Abstract
Purpose
In tissue engineering and regenerative medicine, stem cells are used to produce tissues and organs for both physiological and clinical applications. The interactions among different organs and systems are controlled by chemical and physical stimuli, such as those induced by mesenchymal stem cells (MSC) and photobiomodulation (PBM), respectively. The aim of the present study was to investigate the effects of adipose-derived MSC (AdMSC) and PBM modulations in adult female rat ovaries induced to polycystic ovary syndrome (PCOS), in terms of the number of bone marrow stromal cells obtained and the differentiation of these cells into osteoblasts during 14 days of in vitro culture.
Methods
Bone marrow cells were cultured for 14 days, in the absence and presence of testosterone, prior to analysis of different cellular parameters. The data were analyzed using ANOVA and Fisher’s tests, with statistically significant differences among the means considered for p < 0.05.
Results
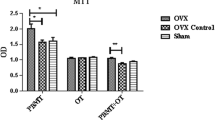
For all groups, the highest mean cell number was obtained after 60 days of in vivo treatments. PBM for 30 days and AdMSC for 60 days positively influenced the cell number before cell differentiation. After 14 days in an osteogenic medium, cell multiplication, cell viability, mineralization, and alkaline phosphatase activity were modulated by the chemical and physical treatments, as well as by testosterone.
Conclusion
The proposed model suggests that ovarian microenvironment modulations induced by mesenchymal stem cells, photobiomodulation, and testosterone can alter the behavior of osteoblasts cultured for 14 days.
Lay Summary
Homeostasis among organs and systems is maintained by complex mechanisms that interact in precisely and continuously ways, controlled by hormones including local autocrine and paracrine signals and systemic endocrine signals. These biomolecules maintain the dynamic balance of organic microenvironments and can be modulated by mesenchymal stem cells. Physical stimuli, such as photobiomodulation, can also modulate tissue functions and the interaction among organs. Hence, knowing the cell microenvironment control mechanisms is an essential aspect of tissue engineering and regenerative medicine.







Similar content being viewed by others
References
Barthes J, Özçelik H, Hindié M, Ndreu-Halili A, Hasan A, Vrana NE. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. Biomed Res Inter. 2014;2014:18. https://doi.org/10.1155/2014/921905
Nurkovic J, Zaletel I, Nurkovic S, Hajrovic S, Mustafic F, Isma J, Skevin AJ, Grbovic V, Filipovic MK, Dolicanin Z. Combined effects of electromagnetic field and low-level laser increase proliferation and later the morphology of human adipose tissue-derived mesenchymal stem cells. Laser Med Sci. 2017;32(1):151–60.
Zhang P, Zhang C, Li J, Han J, Liu X, Yang H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Cell Res Therapy. 2019;10(327):2–13.
Aasebø E, Birkeland E, Sekheim F, Berven F, Brenner AK, Bruserud Ø. The extracellular bone marrow microenvironment – a proteomic comparison of constitutive protein release by in vitro cultured osteoblast and mesenchymal stem cells. Cancer. 2020;13(62):2–24.
Volponi AA, Pang Y, Sharpe PR. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715–22.
Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10:740–9.
Grayson WL, Brunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140–50.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22.
Zheng C, Chen J, Liu S, Jin Y. Stem cell-based bone and dental regeneration: a view of microenvironment modulation. Int J Oral Sci. 2019;11:23.
Lane SW, Williams DA, Watt FM. Modulation the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803.
Loeffler J, Duda GN, Saa FA, Dienelt A. The metabolic microenvironment steers bone tissue regeneration. Trends Endocrinol Metab. 2018;29:99–110.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36.
Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–51.
Gomez-Salazar M, Gonzales-Galofre ZN, Casamitijana J, Crisan M, James AW, Péault B. Five decades later, are mesenchymal stem cells still relevant? Front Bioeng Biotechnol. 2020;8:148.
Patil PSS. A comprehensive review on the role of various materials in the osteogenic differentiation of mesenchymal stem cells with a special focus on the association of heat shock protein and nanoparticles. Cell Tissues Organs. 2014;199(2–3):81–102.
Dehghani SS, Babaee A, Shojaei M, Salehimejad P, Seyedi F, JalalKamali M, Nematollahi-Mahani SN. Different effects of energy dependent irradiation of red and green lights on proliferation of human umbilical cord matrix-derived mesenchymal cells. Laser Med Sci. 2016;31(2):255–61.
Tuby H, Maltz L, Oron U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Laser Surg Med. 2007;39(4):373–8.
Soleimani M, Abbasnia E, Fathi M, Sahrarei H, Fathi Y, Kaka G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neuron and osteoblasts – an in vitro study. Laser Med Sci. 2012;27:423–30.
Wu YH, Wang J, Gong DX, Gu HY, Hu SS, Zhang H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis. Laser Med Sci. 2012;27:509–19.
Horvat-Karajz K, Balogh Z, Kovacs V, Drrernat AH, Srete L, Uher F. In vitro effect of carboplatin, cytarabine, paclitaxel, vincristine, and low-power laser irradiation on murine mesenchymal stem cells. Lasers Surg Med. 2009;41(6):463–9.
Li WT, Leu YC, Wu JL. Red-light light-emitting diode irradiation increase the proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Photomed Laser Surg. 2010;28(Suppl 1):S157–65.
Alves ED, Bonfá ALO, Pigatto GR, Anselmo-Franci JA, Achcar JA, Parizotto NA, Montrezor LH. Photobiomodulation can improve ovarian activity in polycystic ovary syndrome-induced rats. J Photochem Photobiol B Biol. 2019;194:6–13.
Karu T. Mitochondrial signaling in mammalian cells activated by red and near-ir radiation. Photochem Photobiol. 2008;84:1091–9.
Brawer JR, Munoz M, Farookhi R. Development of the polycystic ovarian condition (PCO) in the estradiol valerate-treated rat. Biol Reprod. 1986;35(3):647–55.
Montrezor LH, Carvalho D, Dias MB, Anselmo-Franci JA, Bícego KC, Gargaglioni LH. Hypoxic and hypercapnic ventilatory responses in rats with polycystic ovaries. Resp Physiol Neurobiol. 2015;217:17–24.
Bonfá ALO, Alves ED, Fabricio V, Nonaka KO, Anselmo-Franci JA, Achcar JA, Montrezor LH. Simultaneous alterations in ovaries and bone as a result of Polycystic Ovary Syndrome. Int J Adv Med Biotech. 2020;3(2):2–14.
Wang H. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells Dev. 2012;16(3):467–80.
Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254(2):317–30.
Binder BYK, Sagun JE, Leach JK. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. 2015;11(3):387–93.
Brancaglião LFC, Bonfá ALO, Lemos JES, Rocha NF, Gonçalves VM, Montrezor LH. Effect of ovarian steroids on osteoblast viability and mineralization. Asian J Biol. 2017;2(3):1–18.
Lemos JES, Brancaglião LFC, Bonfá ALO, Rocha NF, Gonçalves VM, Montrezor LH. Effects of culture time, fetal bovine serum, progesterone, testosterone and estradiol on alkaline phosphatase activity, extracellular matrix mineralization and osteoblast viability. The FASEB J. 2018;31(S1):723.6–723.6.
Benevenuto LGD, Achcar JA, Barud HS, Montrezor LH. In vitro study of the effects of bacterial cellulose, glucose, and testosterone on the OSTEO-1 cells multiplication. The FASEB J. 2019;33(S1):602.2–602.2.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65(1–2):55–63.
Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84.
Roy AV. Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin Chem. 1970;16(5):431–6.
Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37(3):99–105.
Wang CY, Liu LN, Zhao ZB. The role of ROS toxicity in spontaneous aneuploidy in cultured cells. Tissue Cell. 2013;45:47–53.
Weinberg RA. The biology of cancer. 2nd ed. New York and London: Garland Press; 2014.
Martín-Romero FJ, Miguel-Lasobras EM, Domínguez-Arroyo JA, Gonzáles-Carrera E, Álvarez IS. Contribution of culture media to oxidative stress and its effect on human oocyte. RBM Online. 2008;17(5):652–61.
Tse SM, Rifas-Shiman SL, Coull BA, Litonjua AA, Oken E, Gold DR. Sex-specific risk factors for childhood wheeze and longitudinal phenotypes of wheeze. J Allergy Clin Immunol. 2016;138:1561-1568.e6.
Regitz-Zagrosek V, Kararigas G. Mechanistic pathway of sex differences in cardiovascular diseases. Physiol Rev. 2017;97:1–37.
Kim JY, Min K, Paik HY, Lee SK. Sex omission and male bias are still widespread in cell experiments. Am J Phsyiol Cell Physiol. 2021;320:C742–9.
Tower J. Sex-specific gene expression and life span regulation. Trends Endocrinol Metab. 2017;28:735–47.
Penaloza C, Estevez B, Orlanski S, Sikorska M, Walker R, Smith C, Smith B, Lockshin RA, Zakeri Z. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J. 2009;23:1869–79.
Klein SL. Immune cells have sex and so should journal article. Endocrinology. 2012;153:2544–50.
Colvard DS, Eriksen EF, Keeting PE, Wilson EM, Lubahn DB, French FS, Riggs BL, Spelsberg TC. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Aca Sci USA. 1989;86:854–7.
Kasperk C, Helmboldt A, Borcsok I, Heuthe S, Cloos O, Niethard F, Ziegler R. Skeletal site-dependent expression of the androgen receptor in human osteoblastic cell populations. Calcif Tissue Int. 1997;61:464–73.
Wiren K, Keenan E, Zhang X, Tamsay B, Orwoll E. Homologous androgen receptor up-regulation in osteoblastic cells may be associated with enhanced functional androgen responsiveness. Endocrinol. 1999;140:3114–24.
Chen J-F, Lin P-W, Tsai Y-R, Yang Y-C, Kang H-Y. Androgen and androgen receptor actions on bone health and disease: from androgen deficiency to androgen therapy. Cells. 2019;8:1318.
Gruber R, Czerwenka K, Wolf F, Ho GM, Willheim M, Peterlik M. Expression of the vitamin D receptor, of estrogen and thyroid hormone receptor alpha- and beta-isoforms, and of the androgen receptor in cultures of native mouse bone marrow and of stroma/osteoblastic cells. Bone. 1999;24:465–73.
Salinas I, Sinha N, Sen A. Androgen-induced epigenic modulations in the ovary. J Endocrinol. 2021;249:R53–64.
Clarke BL, Kholsa S. Androgen and bone. Steroids. 2009;74:296–305.
Boskey AL. Biomineralisation: conflicts, challenges, and opportunities. J Cell Biochem. 1998;30–31:83–91.
Bourne LE, Wheeler-Jones CPD, Orriss IS. Regulation of mineralization in bone and vascular tissue: a comparative review. J Endocrinol. 2021;248(2):R51–65.
Lin PW, Lan KC, Tsai MY, Shyr CR, Chang C, Huang KE, Kang HY. The differential effects of sex hormones on the bone metabolism in mice with androgen receptor deficiency. Adapt Med. 2018;10:143–54.
Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, Barbero A, Manz MG, Martin I. Engineering of a functional bone organ through endochondral ossification. PNAS. 2013;110(10):3997–4002.
Li C, Li G, Liu M, Zhou T, Zhou H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J Biosc Bioengin. 2016;121(2):213–9.
Watt FM, Hogan BL. Out of Eden: stem cell and their niches. Science. 2000;287:1427–30.
Voog J, Jones DL. Stem cell and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–15.
Ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent LEM. Atiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–13.
Mancuso P, Raman S, Glynn A, Barry F, Murphy JM. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Frontier Bioeng Biotechnol. 2018;7:9.
Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low-level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthoped Reumatol. 2015;2(5):1–16.
Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:348–64.
Mosca RC, Ong AA, Albasha O, Bass K, Arany P. Photobiomodulation therapy for wound care: a potent noninvasive, photoceutical approach. Adv Skin Wound Care. 2019;32:157–67.
Tripodi N, Feehan J, Husaric M, Kiatos D, Sidriglou F, Fraser S, Apostolopoulos V. Good, better, best? The effect of polarization on photobiomodulation therapy. J Biphotonics. 2020;13(5):e201960230.
Tripodi N, Corcoran D, Antonello P, Balic N, Caddy D, Knight A, Meehan C, Sidriglou F, Fraser S, Kiatos D, Husaric M, Apostolopoulos V, Feehan J. The effects of photobiomodulation on human dermal fibroblasts in vitro: a systematic review. J Photochem Photobiol B Biol. 2021;214:112100.
Acknowledgements
We thank Renata Aquino de Carvalho for technical support.
Funding
This work was supported by São Paulo Research Foundation (FAPESP), SP, Brazil (Grant # 2016/02811–4).
Author information
Authors and Affiliations
Contributions
Eduardo Donato Alves: methodology, investigation, writing—review and editing. Luíz Guilherme Dércore Benevenuto: methodology, investigation. Bruna Pereira de Morais: methodology, writing—review and editing. Michele Andrade de Barros: methodology. Jorge Alberto Achcar: formal analysis, writing—review and editing. Luís Henrique Montrezor: conceptualization, methodology, investigation, writing—original draft, writing—review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
All the animal experimental procedures were conducted in compliance with the guidelines of the National Council of Control in Animal Experimentation (CONCEA-MCTI, Brazil) and were approved by the local Animal Care and Use Committee (CEUA-UNIARA, nº 030/2016).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, E.D., Benevenuto, L.G.D., Morais, B.P. et al. Ovarian Microenvironment Modulation by Adipose-Mesenchymal Stem Cells and Photobiomodulation Can Alter Osteoblasts Functions In Vitro. Regen. Eng. Transl. Med. 9, 506–517 (2023). https://doi.org/10.1007/s40883-023-00297-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-023-00297-y