Abstract
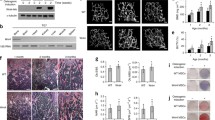
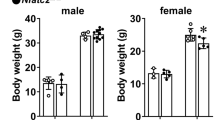
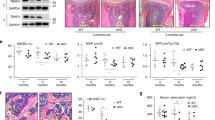
“Senile osteoporosis” is defined as significant aging-associated bone loss and is accompanied by increased fat in the bone marrow. The proportion of adipocytes in the bone marrow is inversely correlated with bone formation and is associated with increased risk of fracture. NF-κB is a transcription factor that functions as a master regulator of inflammation and bone remodeling. NF-κB activity increases during aging; furthermore, constitutive activation of NF-κB significantly impairs skeletal development in neonatal mice. However, the effects of NF-κB activation using a skeletally mature animal model have not been examined. In the current study, an osteoprogenitor (OP)-specific, doxycycline-regulated NF-κB-activated transgenic mouse model (iNF-κB/OP) was generated to investigate the role of NF-κB in bone remodeling in skeletally mature mice. Reduced osteogenesis in the OP-lineage cells isolated from iNF-κB/OP mice was only observed in the absence of doxycycline in vitro. Bone mineral density in the metaphyseal regions of femurs and tibias was reduced in iNF-κB/OP mice. No significant differences in bone volume fraction and cortical bone thickness were observed. Osmium-stained bone marrow fat was increased in epiphyseal and metaphyseal areas in the tibias of iNF-κB/OP mice. These findings suggest that targeting NF-κB activity as a therapeutic strategy may improve bone healing and prevent aging-associated bone loss in aged patients.
Lay Summary
“Senile osteoporosis” denotes significant aging-associated bone loss from the axial and peripheral skeleton and is accompanied by increased fat in the bone marrow. This imbalance in osteogenesis and adipogenesis is associated with an increased incidence of fragility fractures of the spine, hip, knee, shoulder, and wrist. NF-κB is a key regulator of bone remodeling. Increased NF-κB activity was found in many organs during the natural aging process. Clarification of the specific effect of increased NF-κB activity on osteoprogenitors during aging will delineate novel therapeutic approaches to mitigate the adverse effects of chronic inflammation and suppressed bone formation in aging-associated osteoporosis.






Similar content being viewed by others
References
Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55(9):693–8.
Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300. https://doi.org/10.1210/jc.2012-3949.
Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22(6):781–8. https://doi.org/10.1359/jbmr.070315.
Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–64. https://doi.org/10.1359/jbmr.090225.
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21(24):3244–57. https://doi.org/10.1101/gad.1588507.
Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in aging and disease. Aging Dis. 2011;2(6):449–65.
Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A, et al. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J Orthop Res. 2017;35(2):281–8. https://doi.org/10.1002/jor.23270.
Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–9.
Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682–9. https://doi.org/10.1038/nm.1954.
Swarnkar G, Zhang K, Mbalaviele G, Long F, Abu-Amer Y. Constitutive activation of IKK2/NF-kappaB impairs osteogenesis and skeletal development. PLoS One. 2014;9(3):e91421. https://doi.org/10.1371/journal.pone.0091421.
Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95(6):2977–82. https://doi.org/10.1210/jc.2009-2336.
Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–23. https://doi.org/10.1148/radiology.175.1.2315484.
Choi SE, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. J Pathol Transl Med. 2015;49(3):236–42. https://doi.org/10.4132/jptm.2015.03.16.
Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, et al. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng A. 2014;21:875–83. https://doi.org/10.1089/ten.TEA.2014.0144.
Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. https://doi.org/10.1016/j.bone.2014.03.044.
Bermeo S, Gunaratnam K, Duque G. Fat and bone interactions. Curr Osteoporos Rep. 2014;12(2):235–42. https://doi.org/10.1007/s11914-014-0199-y.
Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci U S A. 2013;110(23):9469–74. https://doi.org/10.1073/pnas.1300532110.
Nuttall ME, Shah F, Singh V, Thomas-Porch C, Frazier T, Gimble JM. Adipocytes and the regulation of bone remodeling: a balancing act. Calcif Tissue Int. 2014;94(1):78–87. https://doi.org/10.1007/s00223-013-9807-6.
Schilling T, Kuffner R, Klein-Hitpass L, Zimmer R, Jakob F, Schutze N. Microarray analyses of transdifferentiated mesenchymal stem cells. J Cell Biochem. 2008;103(2):413–33. https://doi.org/10.1002/jcb.21415.
Schilling T, Noth U, Klein-Hitpass L, Jakob F, Schutze N. Plasticity in adipogenesis and osteogenesis of human mesenchymal stem cells. Mol Cell Endocrinol. 2007;271(1–2):1–17. https://doi.org/10.1016/j.mce.2007.03.004.
Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18(9):980–2. https://doi.org/10.1096/fj.03-1100fje.
Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M, et al. Loss of Gs alpha early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J Bone Miner Res. 2014;29(11):2414–26. https://doi.org/10.1002/jbmr.2270.
Gao B, Huang Q, Lin YS, Wei BY, Guo YS, Sun Z, et al. Dose-dependent effect of estrogen suppresses the osteo-adipogenic transdifferentiation of osteoblasts via canonical Wnt signaling pathway. PLoS One. 2014;9(6):e99137. https://doi.org/10.1371/journal.pone.0099137.
Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, et al. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng A. 2015;21(5–6):875–83. https://doi.org/10.1089/ten.TEA.2014.0144.
Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. https://doi.org/10.7150/ijbs.2929.
Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100(11):2697–713. https://doi.org/10.1172/JCI119815.
Torti FM, Torti SV, Larrick JW, Ringold GM. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J Cell Biol. 1989;108(3):1105–13.
Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. https://doi.org/10.1242/dev.02480.
Tella SH, Gallagher JC. Biological agents in management of osteoporosis. Eur J Clin Pharmacol. 2014;70(11):1291–301. https://doi.org/10.1007/s00228-014-1735-5.
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–33. https://doi.org/10.1001/jama.2016.11136.
Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev. 2016;99(Pt A:12–27. https://doi.org/10.1016/j.addr.2015.10.005.
Dang L, Liu J, Li F, Wang L, Li D, Guo B, et al. Targeted delivery systems for molecular therapy in skeletal disorders. Int J Mol Sci. 2016;17(3):428. https://doi.org/10.3390/ijms17030428.
Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med. 2012;18(2):307–14. https://doi.org/10.1038/nm.2617.
Liang C, Guo B, Wu H, Shao N, Li D, Liu J, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med. 2015;21(3):288–94. https://doi.org/10.1038/nm.3791.
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125(4):1509–22. https://doi.org/10.1172/JCI77716.
Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17(6):963–76. https://doi.org/10.1359/jbmr.2002.17.6.963.
Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, et al. Osterix-Cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8(8):e71318. https://doi.org/10.1371/journal.pone.0071318.
Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14(1):78–84. https://doi.org/10.1200/JCO.1996.14.1.78.
Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F, et al. Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. Int J Mol Sci. 2017;18(6). https://doi.org/10.3390/ijms18061264.
Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. https://doi.org/10.1016/j.cmet.2010.12.008.
Funding
This work was supported by NIH grant 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, T., Pajarinen, J., Kohno, Y. et al. Increased NF-κB Activity in Osteoprogenitor-Lineage Cells Impairs the Balance of Bone Versus Fat in the Marrow of Skeletally Mature Mice. Regen. Eng. Transl. Med. 6, 69–77 (2020). https://doi.org/10.1007/s40883-019-00112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00112-7