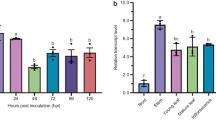
Abstract
Cassava anthracnose disease (CAD), caused by Colletotrichum gloeosporioides f. sp. manihotis, is one of the most important diseases that cause significant yield loss in cassava. Recently, involvement of microRNAs (miRNAs), a class of small, single-stranded, non-coding RNAs, in the resistance against anthracnose disease has been indicated. In this study, two cassava cultivars that have different degree of CAD susceptibility were utilized to investigate the differences in defense responses these cultivars exhibited and to examine the role of seven miRNA families (mes-MIR156, 159, 164, 171, 396, 408, and 530) during CAD infection. Unlike the susceptible cultivar, the tolerant cultivar responded to fungal attack in the forms of hypersensitive response at the primary site of infection (or stem), as well as systemic induction of different defensive measures in the distal organs (or leaves) such as callose deposition, H2O2 accumulation, and upregulated expression of the miRNAs being studied. Two of the miRNAs, mes-MIR156 and mes-MIR164, were able to move across the kingdom boundary to the invading fungal cells. With the availability of genome sequence of C. gloeosporioides strain Cg-14, the mes-MIR156 and mes-MIR164 were predicted to target five and eleven fungal genes, respectively. Based on the differences in defense responses observed in the CAD-tolerant and CAD-susceptible cultivars, we then propose that the tolerant cultivar possesses a distinct defense mechanism against C. gloeosporioides f. sp. manihotis infection. In this defense mechanism, certain miRNAs are needed to help protect the host plant from the invading fungal pathogen.







Similar content being viewed by others
Data availability
The dataset generated during and/or analyzed during the current study are not publicly available due to the data ownership policy of the institutions the authors belong but are available from the corresponding author on reasonable request.
References
Alvarez ME, Pennell RI, Meijer P, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, Edsinger-Gonzales E, Grimwood J, Schmutz J, Rabbi IY, Egesi C, Nauluvula P, Lebot V, Ndunguru J, Mkamilo G, Bart RS, Setter TL, Gleadow RM, Kulakow P, Ferguson ME, Rounsley S, Rokhsar DS (2016) Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nature Biotechnology 34:562–570
Cai Q, Qiao L, Wang M, He B, Lin F, Palmquist J, Huang S, Jin H (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360:1126–1129
Cano J, Guarro J, Gend J (2004) Molecular and morphological identification of Colletotrichum species of clinical interest. Journal of Clinical Microbiology 42:2450–2454
Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proceedings of the National Academy of Sciences of the Unites States of America 102:3459–3464
Chen X, Zhang Z, Liu D, Zhang K, Li A, Mao L (2010) SQUAMOSA promoter-binding protein-like transcription factors; star players for plant growth and development. Journal of Integrative Plant Biology 52:946–951
Cheng H, Gao J, An Z, Huang H (2010) A rapid method for isolation of low-molecular-weight RNA from Arabidopsis using low salt concentration buffer. Integrative Journal of Plant Biology 1:e14
Choi J, Kim K, Jeon J, Wu J, Song H, Asiegbu FO, Lee Y (2014) funRNA: a fungi-centered genomics platform for genes encoding key components of RNAi. BMC Genomics 15:514
Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Research 39:W155–W159
Daudi A, O’Brien JA (2012) Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-protocol 2:e263
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15
Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, Somervilles SC, Voigt CA (2013) Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiology 161:1433–1444
Feng H, Duan X, Zhang Q, Li X, Wang B, Huang L, Wang X, Kang Z (2014) The target gene of tae-miR164, a novel NAC transcription factor from the NAM family, negatively regulates resistance of wheat to stripe rust. Molecular Plant Pathology 15:284–296
Fokunang CN, Akem CN, Ikotun T, Dixon AGO, Tembe EA (2000) Role of the insect vector, Pseudotheraptus devastans, in cassava anthracnose disease development. European Journal of Plant Pathology 106:319–237
Fokunang CN, Dixon AGO, Ikotun T, Tembe EA, Akem CN, Asiedu R (2001) Anthracnose: an economic disease of cassava in Africa. Pakistan Journal of Biological Science 4:920–925
Gacek-Matthews A, Berger H, Sasaki T, Wittstein K, Gruber C, Lewis ZA, Strauss J (2016) KdmB, a jumonji histone H3 demethylase, regulates genome-wide H3K4 trimethylation and is required for normal induction of secondary metabolism in Aspergillus nidulans. PLoS Genetics 12:e1006222
Gundersen GG, Worman HJ (2013) Nuclear positioning. Cell 152:1376–1389
Gupta OP, Sharma P, Gupta RK, Sharma I (2014) Current status on role of miRNAs during plant-fungus interaction. Physiological and Molecular Plant Pathology 85:1–7
Hagen I, Yanagida M (1995) The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. Journal of Cell Biology 129:1033–1047
Hahn SK, Ikotun T, Theberge RL, Swennen R (1989) Major economic diseases of cassava, plantains and cooking starchy bananas in Africa. Tropical Agricultural Research Series 22:106–112
Hou J, Feng H, Chang H, Liu Y, Li G, Yang S, Sun C, Zhang M, Yuan Y, Sun J, Zhu-Salzman K, Zhang H, Qin Q (2020) The H3K4 demethylase Jar1 orchestrates ROS production and expression of pathogenesis-related genes to facilitate Botrytis cinerea virulence. New Phytologist 225:930–947
Howe KL, Contreras-Moreira B, De Silva N, Maslen G, Akanni W, Allen J, Alvarez-Jarreta J, Barba M, Bolser DM, Cambell L, Carbajo M, Chakiachvili M, Christensen M, Cummins C, Cuzick A, Davis P, Fexova S, Gall A, George N, Gil L, Gupta P, Hammond-Kosack KE, Haskell E, Hunt SE, Jaiswal P, Janacek SH, Kersey PJ, Langridge N, Maheswari U, Maurel T, McDowall MD, Moore B, Muffato M, Naamati G, Naithani S, Olson A, Papatheodorou I, Patricio M, Paulini M, Pedro H, Perry E, Preece J, Rosello M, Russell M, Sitnik V, Staines DM, Stein J, Tello-Ruiz MK, Trevanion ST, Urban M, Wei S, Ware D, Williams G, Yates AD, Flicek P (2020) Ensembl Genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Research 48:D689–D695
Hu G, Lei Y, Liu J, Hao M, Zhang Z, Tang Y, Chen A, Wu J (2020) The ghr-miR164 and GhNAC100 modulate cotton plant resistance against Verticillium dahlia. Plant Science 293:110438
Iwakawa H, Tomari Y (2015) The functions of microRNAs: mRNA decay and translational repression. Trends in Cell Biology 25:651–665
Kim JW, Shim SH (2019) The fungus Colletotrichum as a source for bioactive secondary metabolites. Archives of Pharmacal Research 42:735–753
Klein J, Saedler H, Huijser P (1996) A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus flower meristem identity gene SQUAMOSA. Molecular and General Genetics 250:7–16
Klemm E, Ninnemann H (1978) Correlation between absorbance changes and a physiological response induced by blue light in Neurospora crassa. Photochemistry and Photobiology 28:227–230
Kunkeaw S, Worapun J, Smith DR, Triwitayakorn K (2010) An in vitro detached leaf assay for pre-screening resistance to anthracnose disease in cassava (Manihot esculenta Crantz). Australasian Plant Pathology 39:547–550
Lee SC, Hwang BK (2005) Induction of some defense-related genes and oxidative burst is required for the establishment of systemic acquired resistance in Capsicum annuum. Planta 221:790–800
Lertpanyasampatha M, Viboonjun U, Kongsawadworakul P, Chrestin H, Narangajavana J (2014) Differential expression of microRNAs and their targets reveals a possible dual role in physiological bark disorder in rubber tree. Journal of Plant Physiology 171:1117–1126
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research 30:325–327
Li B, Meng X, Shan L, He P (2016) Transcriptional regulation of pattern-triggered immunity in plants. Cell Host & Microbe 19:641–650
Liu Y, He Q, Cheng P (2003) Photoreception in Neurospora: a tale of two White Collar proteins. Cellular and Molecular Life Sciences 60:2131–2138
Liu M, Shi Z, Zhang X, Wang M, Zhang L, Zheng K, Liu J, Hu X, Di C, Qian Q, He Z, Yng D (2019) Inducible overexpression of Ideal Plant Architecture1 improves both yield and disease resistance in rice. Nature Plants 5:389–400
Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2011) Callose deposition: a multifaceted plant defense response. Molecular Plant-Microbe Interactions 24:183–193
Mahto BK, Singh A, Pareek M, Rajam MV, Dhar-Ray S, Reddy PM (2020) Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato. Plant Molecular Biology 104:381–395
Malone CJ, Fixsen WD, Moritz R, Han M (1999) UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126:3171–3181
Marzluf GA (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiology and Molecular Biology Reviews 61:17–32
McCallum EJ, Anjanappa RB, Gruissem W (2017) Tacking agriculturally relevant diseases in the staple crop cassava (Manihot esculenta). Current Opinion in Plant Biology 38:50–58
Moraga J, Gomes W, Pinedo C, Cantoral JM, Hanson JR, Carbu M, Garrido C, Durán-Patrón R, Collada IG (2019) The current status on secondary metabolites produced by plant pathogenic Colletotrichum species. Phytochemistry Reviews 18:215–239
Nakashima J, Laosinchai W, Cui X, Brown RM (2003) New insight into the mechanism of cellulose and callose biosynthesis; protease may regulate callose biosynthesis upon wounding. Cellulose 10:369–389
Nedukha OM (2015) Callose: localization, functions, and synthesis in plant cells. Cytology and Genetics 49:49–57
O’Brien JA, Daudi A, Butt VS, Bolwell GP (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779
Owolade OF, Dixon AGO, Adeoti AA, Osunlaja SO (2005) Sources of resistance to cassava anthracnose disease. African Journal of Biotechnology 4:570–572
Panwar SL, Moye-Rowley WS (2006) Long chain base tolerance in Saccharomyces cerevisiae is induced by retrograde signals from the mitochondria. Journal of Biological Chemistry 281:6376–6384
Perfect SE, Hughes HB, O’Connell RJ, Green JR (1999) Colletotrichum: a model genus for studies on pathology and fungal-plant interactions. Fungal Genetics and Biology 27:186–198
Perino EHB, Glienke C, Silva AO, Deising HB (2020) Molecular characterization of the purine degradation pathways genes ALA1 and URE1 of the maize anthracnose fungus Colletotrichum graminicola identified urease as a novel target for plant disease control. Phytopathology 110:1530–1540
Pinweha N, Asvarak T, Viboonjun U, Narangajavana J (2015) Involvement of miR160/miR393 and their targets in cassava responses to anthracnose disease. Journal of Plant Physiology 174:26–35
Piršelová B, Matušíková I (2013) Callose: the plant cell wall polysaccharide with multiple biological functions. Acta Physiologiae Plantarum 35:635–644
Riddell RW (1950) Permanent stained mycological preparations obtained by slide culture. Mycologia 42:265–270
Saelim L, Phansiri S, Suksangpanomrung M, Netrphan S, Narangajavana J (2009) Evaluation of a morphological marker selection and excision system to generate marker-free transgenic cassava plants. Plant Cell Reports 28:445–455
Scalschi L, Llorens E, Camañes G, Pastor V, Fernández-Crespo E, Flors V, García-Agustín P, Vicedo B (2015) Quantification of callose deposition in plant leaves. Bio-protocol 5:e1610
Schjoerring JK, Husted S, Mack G, Mattsson M (2002) The regulation of ammonium translation in plants. Journal of Experimental Botany 53:883–890
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Developmental Cell 8:517–527
Solomon PS, Oliver RP (2001) The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 213:241–249
Stief A, Altmann S, Hoffmann K, Pant BD, Scheible W-R, Baurle I (2014) Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 26:1792–1807
Tetyuk O, Benning UF, Hoffmann-Benning S (2013) Collection and analysis of Arabidopsis phloem exudates using the EDTA-facilitated method. Journal of Visualized Experiments 80:e51111
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3-new capabilities and interfaces. Nucleic Acids Research 40:e115
Valle EM, Boggio SB, Heldt HW (1998) Free amino acid composition of phloem sap and growing fruit of Lycopersicon esculentum. Plant and Cell Physiology 39:458–461
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Walker SE, Lorsch J (2013) RNA purification - precipitation methods. Methods in Enzymology 530:337–343
Wang M, Weiberg A, Lin F, Thomma BPHJ, Huang H, Jin H (2016) Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nature Plants 2:16151
Wang B, Sun Y, Song N, Zhao M, Liu R, Feng H, Wang X, Kang Z (2017) Puccinia striiformis f. sp. tritici microRNA-like RNA1 (Pst-milR1), and important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytologist 215:338–350
Wang Z, Xia Y, Lin S et al (2018) Osa-miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Plant Journal 95:584–597
Wu G, Poethig RS (2006) Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133:3539–3547
Wu L, Chen H, Curtis C, Fu ZQ (2014) Go in for the kill: how plants deploy effector-triggered immunity to combat pathogens. Virulence 5:710–721
Yin H, Hong G, Li L, Zhang X, Kong Y, Sun Z, Li J, Chen J, He Y (2019) miR156/SPL9 regulates reactive oxygen species accumulation and immune response in Arabidopsis thaliana. Phytopathology 109:612–642
Yu N, Niu Q, Ng K, Chua N (2015) The role of miR156/SPLs modules in Arabidopsis lateral root development. Plant Journal 83:673–685
Zhang T, Zhao Y, Zhao J, Wang S, Jin Y, Chen Z, Fang Y, Hua C, Ding S, Guo H (2016) Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nature Plants 2:16153
Zhang Q, Li Y, Zhang Y, Wu C, Wang S, Hao L, Wang S, Li T (2017) Md-miR156ab and Md-miR395 target WRKY transcription factors to influence apple resistance to leaf spot disease. Frontiers in Plant Science 8:526
Zhang L, Li Y, Zheng Y, Wang H, Yang X, Chen J, Zhou S, Wang L, Li X, Ma X, Zhao J, Pu M, Feng H, Fan J, Zhang J, Huang Y, Wang W (2020) Expression a target mimic of miR156fhl-3p enhances rice blast disease resistance without yield penalty by improving SPL14 expression. Frontiers in Genetics 11:327
Zhou G, Zhou Y, Chen X (2017) New insight into inter-kingdom communication: horizontal transfer of mobile small RNAs. Frontiers in Microbiology 8:768
Zipfel C, Felix G (2005) Plants and animals: a different taste for microbes? Current Opinion in Plant Biology 8:353–360
Acknowledgements
This research work was supported by the Center of Excellence on Agricultural Biotechnology, Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (AG-BIO/MHESI). A scholarship from the Thailand Graduate Institute of Science and Technology (TGIST), National Science and Technology Development Agency, Thailand, was gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
JN conceived and designed experiments. NP conducted experiments. P. Sae-Lim analyzed the results. SN, JN, P. Sojikul, and UV discussed the results. SN wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40858_2022_503_MOESM1_ESM.doc
Supplementary file1 (DOC 569 KB) Recovery of fungal hyphae from the stems of CAD-infected HB60 and HN. After 4 days of the introduction of C. gloeosporioides f. sp. manihotis spore suspension to HB60 and HN planlets through the puncture wound (black arrow), the stems of CAD-infected plants were cut into pieces and labeled A to E depending on their location either above (A, B and C) or below (D, E and F) the infected point (A). The cut pieces of stems were then placed on PDA plates and incubated at 30°C for 5 days (B). Fungal growth was observed only from the stems of plants infected with C. gloeosporioides f. sp. manihotis but not from those exposed to sterile water (control).
40858_2022_503_MOESM3_ESM.doc
Supplementary file3 (DOC 216 KB) Specificity of the primers used in qRT-PCR. Each experiment was performed in triplicate. Based on the melting or dissociation curves, amplification of the genes of interest with selected primer pairs yielded only a single amplicon.
40858_2022_503_MOESM4_ESM.doc
Supplementary file4 (DOC 398 KB) Schematic maps of putative cis-acting elements identified by in silico analysis of the 3-kb upstream sequence of seven miRNA gene families and their target genes in cassava. Positions are with respect to the first base of the translation start site (ATG).
40858_2022_503_MOESM5_ESM.xlsx
Supplementary file5 (XLSX 16 KB) Radial growth of C. gloeosporioides f. sp. manihotis on PDA plates spread with various types of cassava phloem exudates
40858_2022_503_MOESM6_ESM.doc
Supplementary file6 (DOC 62 KB) Mapping of mes-MIR156 and mes-MIR164 with predicted target transcripts of C. gloeosporioides Cg-14 (GCA_000446055). The mes-MIR156 and mes-MIR164-guided cleavage sites were predicted after the 10th position of the miRNA 5´ end (arrows). Watson-Crick pairing (:) and wobble pairing (.) are indicated.
Rights and permissions
About this article
Cite this article
Pinweha, N., Netrphan, S., Sojikul, P. et al. Cross-kingdom microRNA transfer for the control of the anthracnose disease in cassava. Trop. plant pathol. 47, 362–377 (2022). https://doi.org/10.1007/s40858-022-00503-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-022-00503-2