Abstract
A loop-mediated isothermal amplification (LAMP) assay was developed and compared to polymerase chain reaction (PCR), nested PCR, and selective isolation assays for detection of Xanthomonas albilineans, the causal agent of sugarcane leaf scald. The pathogen was isolated on selective medium from 44 out of 45 (98%) samples taken from symptomatic stalks, and from 44 out of 70 (63%) samples from asymptomatic stalks that were collected from plots with symptomatic stalks. Forty-two (93%), 41 (91%), and 42 (93%) symptomatic samples tested positive by LAMP, PCR and nested PCR, respectively. The pathogen was detected in 19 (27%), 8 (11%), and 25 (36%) of the 70 asymptomatic samples by LAMP, PCR and nested PCR, respectively. Symptomatic stalks were mainly, but not always, associated with high populations of the pathogen (107–109 CFU/ml), and asymptomatic stalks with low populations (<103 CFU/ml) or no bacteria. Although our LAMP and nested PCR methods detected 10 CFU/ml of X. albilineans in suspensions prepared with pure culture, they sometimes failed to detect the pathogen in samples with low pathogen populations. Isolation on selective medium along with another method should therefore be used for detection of the pathogen in asymptomatic stalks, especially in quarantine programs.

Similar content being viewed by others
References
Alvarez AM, Schenck S, Benedict AA (1996) Differentiation of Xanthomonas albilineans strains with monoclonal antibody reaction patterns and DNA fingerprints. Plant Pathology 45:358–366
Amata RL, Fernandez E, Filloux D, Martin D, Rott P, Roumagnac P (2016) Prevalence of Sugarcane yellow leaf virus in sugarcane producing regions in Kenya revealed by reverse transcription loop-mediated isothermal amplification method. Plant Disease 100:260–268
Ash GJ, Lang JM, Triplett LR, Stodart BJ, Verdier V, Vera Cruz C, Rott P, Leach JE (2014) Development of a genomics-based LAMP (loop-mediated isothermal amplification) assay for detection of Pseudomonas fuscovaginae from rice. Plant Disease 98:909–915
Birch RG (2001) Xanthomonas albilineans and the antipathogenesis approach to disease control. Molecular Plant Pathology 2:1–11
Bühlmann A, Pothier JF, Rezzonico F, Smits THM, Andreou M, Boonham N, Duffy B, Frey JE (2013) Erwinia amylovora loop-mediated isothermal amplification (LAMP) assay for rapid pathogen detection and on-site diagnosis of fire blight. Journal of Microbiological Methods 92:332–339
Champoiseau P, Daugrois J-H, Girard J-C, Royer M, Rott PC (2006) Variation in albicidin biosynthesis genes and in pathogenicity of Xanthomonas albilineans, the sugarcane leaf scald pathogen. Phytopathology 96:33–45
Chandra A, Keizerweerd AT, Que Y, Grisham M (2015) Loop-mediated isothermal amplification (LAMP) based detection of Colletotrichum falcatum causing red rot in sugarcane. Molecular Biology Reports 42:1309–1316
Cochran WG (1950) The comparison of percentages in matched samples. Biometrika 37:256–266
Cociancich S, Pesic A, Uhlmann S, Petras D, Kretz J, Schubert V, Vieweg L, Duplan S, Marguerettaz M, Noëll J, Pieretti I, Hügelland M, Kemper S, Rott P, Royer M, Süssmuth RD (2015) The gyrase inhibitor albicidin consists of Para-aminobenzoic acids and cyanoalanine. Nature Chemical Biology 11:195–197
Comstock JC (2001) Foliar symptoms of sugarcane leaf scald. Sugar Journal 64:23–32
Comstock JC, Irey MS (1992) Detection of the sugarcane leaf scald pathogen Xanthomonas albilineans, using tissue blot immunoassay, ELISA, and isolation techniques. Plant Disease 76:1033–1035
Daugrois JH, Boisne-Noc R, Champoiseau P, Rott P (2012) The revisited infection cycle of Xanthomonas albilineans, the causal agent of leaf scald of sugarcane. Functional Plant Science and Biotechnology 6 (Special issue 2):91–97
Davis MJ, Rott P, Baudin P, Dean JL (1994) Evaluation of selective media and immunoassays for detection of Xanthomonas albilineans, causal agent of sugarcane leaf scald disease. Plant Disease 78:78–82
Davis MJ, Rott P, Astua-Monge G (1998). Nested, multiplex PCR for detection of both Clavibacter xyli subsp. xyli and Xanthomonas albilineans in sugarcane. In: Offered papers abstracts, Vol. 3, 7th International congress of plant pathology, Edinburgh, Scotland, 9–16 August 1998. Edinburgh, International Society of Plant Pathology, abstract 3.3.4
Fischbach J, Xander NC, Frohme M, Glökler JF (2015) Shining a light on LAMP assays – a comparison of LAMP visualization methods including the novel use of berberine. Biotechnology Techniques 58:189–194
Garces FF, Gutierrez A, Hoy J (2014) Detection and quantification of Xanthomonas albilineans by qPCR and potential characterization of sugarcane resistance to leaf scald. Plant Disease 98:121–126
Ghai M, Singh V, Martin LA, McFarlane SA, van Antwerpen T, Rutherford S (2014) A rapid and visual loop-mediated isothermal amplification assay to detect Leifsonia xyli subsp. xyli targeting a transposase gene. Letters in Applied Microbiology 59:648–657
Goto M, Honda E, Ogura A, Nomoto A, Hanaki K (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechnology Techniques 46:167–172
Guinet-Brial I, Girard JC, Roumagnac P, Daugrois JH, Fernandez E, Rott P (2013) Visacane: an innovative quarantine tool for the exchange of pest and disease-free sugarcane germplasm. Proceedings International Society of Sugar Cane Technologists 28:1150–1162
Jaufeerally-Fakim Y, Autrey JC, Toth I, Daniels M, Dookun A (2002) Amplification polymorphism among Xanthomonas albilineans strains, using a single oligonucleotide primer. European Journal of Plant Pathology 108:121–130
Keizerweerd AT, Chandra A, Grisham MP (2015) Development of a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of Sugarcane mosaic virus and Sorghum mosaic virus in sugarcane. Journal of Virological Methods 212:23–29
Lang JM, Langlois P, Nguyen MHR, Triplett LR, Purdie L, Holton TA, Djikeng A, Vera Cruz CM, Verdier V, Leach JE (2014) Sensitive detection of Xanthomonas oryzae pathovars oryzae and oryzicola by loop-mediated isothermal amplification. Applied and Environmental Microbiology 80:4519–4530
Lenarčič R, Morisset D, Pirc M, Llop P, Ravnikar M, Dreo T (2014) Loop-mediated isothermal amplification of specific endoglucanase gene sequence of the bacterial wilt pathogen Ralstonia solanacearum. PLoS One 9:e96027
Liu J, Xu L, Guo J, Chen R, Grisham MP, Que Y (2013) Development of loop-mediated isothermal amplification for detection of Leifsonia xyli subsp. xyli in sugarcane. BioMed Research International 2013:357692
López MM, Llop P, Olmos A, Marco-Noales E, Cambra M, Bertolini E (2009) Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Current Issues in Molecular Biology 11:13–46
Martin JP, Carpenter CW, Weller DM (1932) Leaf scald disease of sugarcane in Hawaii. Hawaiian Planters Records 36:145–196
Mensi I, Vernerey MS, Gargani D, Nicole M, Rott P (2014) Breaking dogmas: the plant vascular pathogen Xanthomonas albilineans is able to invade non-vascular tissues despite its reduced genome. Open Biology 4:130116
Miller SA, Beed FD, Lapaire Harmon C (2009) Plant disease diagnostic capabilities and networks. Annual Review of Phytopathology 47:15–38
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochemical and Biophysical Research Communications 289:150–154
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Molecular and Cellular Probes 16:223–229
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Research 28:e63
Orian G (1942) Artificial hosts of the sugarcane leaf scald organism. Revue Agricole et Sucrière de Ile Maurice 21:285–304
Orian G (1962) A disease of Paspalum dilatatum in Mauritius caused by a species of bacterium closely resembling Xanthomonas albilineans (Ahby) Dowson. Revue Agricole et Sucrière de Ile Maurice 41:7–20
Pan Y-B, Grisham MP, Burner DM, Legendre BL, Wei Q (1999) Development of polymerase chain reaction primers highly specific for Xanthomonas albilineans, the causal bacterium of sugarcane leaf scald disease. Plant Disease 83:218–222
Pieretti I, Royer M, Barbe V, Carrere S, Koebnik R, Cociancich S, Couloux A, Darrasse A, Gouzy J, Jacques M-A, Lauber E, Manceau C, Mangenot S, Poussier S, Segurens B, Verdier V, Arlat M, Rott P (2009) The complete genome of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics 10:616
Pieretti I, Bolot S, Carrère S, Barbe V, Cociancich S, Rott P, Royer M (2015a) Draft genome sequence of Xanthomonas sacchari strain LMG 476. Genome Announcements 3:e00146-15
Pieretti I, Cociancich S, Bolot S, Carrère S, Morisset A, Rott P, Royer M (2015b) Full genome sequence analysis of two isolates reveals a novel Xanthomonas species close to the sugarcane pathogen Xanthomonas albilineans. Genes 6:714–733
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ricaud C, Ryan CC (1989) Leaf scald. In: Ricaud C, Egan BT, Gillaspie Jr. AG, Hughes CG (Eds.) Diseases of sugarcane. Major diseases. Amsterdam, The Netherlands. Elsevier. pp. 39–53
Rigano LA, Marano MR, Castagnaro AP, Morais Do Amaral A, Vojnov A (2010) Rapid and sensitive detection of citrus bacterial canker by loop-mediated isothermal amplification combined with simple visual evaluation methods. BMC Microbiology 10:176–184
Rott P, Davis MJ (2000) Leaf scald. In: Rott P, Bailey RA, Comstock JC, Croft BJ, Saumtally AS (eds) A guide to sugarcane diseases. La Librairie du Cirad, Montpellier, pp 38–44
Rott P, Davis MJ, Baudin P (1994) Serological variability in Xanthomonas albilineans, causal agent of leaf scald disease of sugarcane. Plant Pathology 43:344–349
Rott P, Mohamed IS, Klett P, Soupa D, de Saint-Albin A, Feldmann P, Letourmy P (1997) Resistance to leaf scald disease is associated with limited colonization of sugarcane and wild relatives by Xanthomonas albilineans. Phytopathology 87:1202–1213
Saharan P, Dhingolia S, Khatri P, Duhan JS, Gahlawat SK (2014) Loop-mediated isothermal amplification (LAMP) based detection of bacteria: a review. African Journal of Biotechnology 13:1920–1928
Temple TN, du Toit LJ, Derie ML, Johnson KB (2013) Quantitative molecular detection of Xanthomonas hortorum pv. carotae in carrot seed before and after hot-water treatment. Plant Disease 97:1585–1592
Wang ZK, Comstock JC, Hatziloukas E, Schaad NW (1999) Comparison of PCR, BIO-PCR, DIA, ELISA and isolation on semiselective medium for detection of Xanthomonas albilineans, the causal agent of leaf scald of sugarcane. Plant Pathology 48:245–252
Acknowledgements
Vanessa Duarte Dias was supported by a fellowship from the Brazil Scientific Mobility Program sponsored by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). We thank Dr. Alexandre S. G. Coelho for his help in the statistical analysis of data. This work would not have been possible without the financial support of the Florida Sugar Cane League. This work is supported by the USDA National Institute of Food and Agriculture (project Hatch/Rott FLA-BGL-005404).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Jorge T. De Souza
Electronic supplementary material
Supplementary Fig. 1
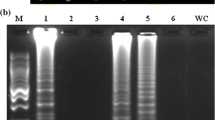
Detection of Xanthomonas albilineans by PCR (A) and nested PCR (B) in water suspensions from a pure culture of the pathogen (10-fold dilutions). Lane 1 = 100 bp ladder; lanes 2 to 8 = 106, 105, 104, 103, 102, 10, and 1 CFU/ml, respectively. The sizes of the PCR (A) and nested PCR (B) amplicons are 440 and 308 bp, respectively (PPTX 339 kb)
Supplementary Table 1
Characteristics of the 115 sugarcane samples used in this study (DOCX 19 kb)
Supplementary Table 2
Primers used to detect Xanthomonas albilineans by loop-mediated isothermal amplification (LAMP), polymerase chain reaction (PCR) and nested polymerase chain reaction (Nested PCR) (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Duarte Dias, V., Fernandez, E., Cunha, M.G. et al. Comparison of loop-mediated isothermal amplification, polymerase chain reaction, and selective isolation assays for detection of Xanthomonas albilineans from sugarcane. Trop. plant pathol. 43, 351–359 (2018). https://doi.org/10.1007/s40858-018-0216-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-018-0216-2