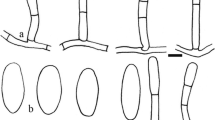
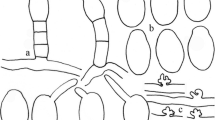
Abstract
Records of Golovinomyces species new to Thailand are described on the hosts Ageratum conyzoides, Bidens pilosa, Dahlia pinnata, D. × hortensis, Helianthus annuus, Lactuca indica, Laggera crispata, Sonchus oleraceus (Asteraceae), Lygisma inflexum (Asclepiadaceae), Myosotis scopioides (Boraginaceae), Coccinia indica, Coccinia grandis (Cucurbitaceae), Vigna umbellata (Fabaceae), Torenia fournieri (Linderniaceae), Plantago major (Plantaginaceae) and Verbena × hybrida (Verbenaceae). The identifications of the particular Golovinomyces species have been performed by means of morphological examinations supplemented by molecular sequence analyses. On the basis of molecular analyses, the powdery mildew on Ocimum tenuiflorum (Lamiaceae) proved to represent a species of its own, which is referred to as Golovinomyces ocimi comb. nov. The application of Oidium ocimi, the basionym of this combination, is determined by lecto- and epitypification. Lygisma inflexum, Laggera crispata and Vigna umbellata are new host records for Golovinomyces worldwide.
















Similar content being viewed by others
References
Amano HK (1986) Host range and geographical distribution of the powdery mildew fungi. Japan Scientific Societies Press, Tokyo
Bappammal M, Hosagoudar VB, Udaiyan K (1995) Powdery mildews of Tamil Nadu, India. New Botanist 22:81–175
Braun U (1987) A monograph of the Erysiphales (powdery mildews). Beihefte zur Nova Hedwigia 89:1–700
Braun U, Cook RTA (2012) Taxonomic manual of the Erysiphales (powdery mildews). CBS biodiversity. Ser. No. 11. CBS–KNAW fungal biodivers. Centre, Utrecht
Braun U, Takamatsu S (2000) Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences – some taxonomic consequences. Schlechtendalia 4:1–33
Cho SE, Choi YJ, Han KS, Park MJ, Shin HD (2016) First report of powdery mildew caused by Golovinomyces orontii on Lactuca sativa in Korea. Plant Disease 100:1015
Dugan F (2013) Golovinomyces spadiceus causes powdery mildew on Coreopsis hybrida ‘full moon’ (Heliantheae, Asteraceae) in Washington State. North American Fungi 8:1–3
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32:1792–1797
Farr DF, Rossman AY (2017) Fungal Databases, Syst. Mycol. and Microbiol. Lab., ARS, USDA. Available at: http://nt.ars-grin.gov/fungaldatabases/. Accessed 8 June 2017
Felsenstein J (1985) Confidence limits on phylogenetics: an approach using the bootstrap. Evolution 39:783–791
Hirata T, Takamatsu S (1996) Nucleotide sequence diversity of rDNA internal transcribed spacers extracted from conidia and cleistothecia of several powdery mildew fungi. Mycoscience 37:283-288
Hosagoudar VB, Agarwal DK (2009) Powdery mildews of India–check list. Associated Publishing Company, New Delhi
Matsuda S, Takamatsu S (2003) Evolution of host-parasite relationships of Golovinomyces (Ascomycetes: Erysiphaceae) inferred from nuclear rDNA sequences. Molecular Phylogenetics and Evolution 27:314–327
Meeboon J, Takamatsu S (2015a) Erysiphe takamatsui, a powdery mildew of lotus: rediscovery of teleomorph after 40 years, morphology and phylogeny. Mycoscience 56:159–167
Meeboon J, Takamatsu S (2015b) Erysiphe viburni-plicati and Podosphaera photiniae, two new species of Erysiphales (Ascomycota) from Japan. Mycoscience 56:14–23
Meeboon J, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand II. Erysiphe species on Anacardiaceae, Apocynaceae, Araliaceae, Aristolochiaceae, Bixaceae, Brassicaceae, Caprifoliaceae, Cleomaceae, Convolvulaceae, Cucurbitaceae and Euphorbiacea. Tropical Plant Pathology 41:357–369
Meeboon J, Takamatsu S (2017a) Notes on powdery mildews (Erysiphales) in Thailand III. Erysiphe species on Fabaceae, Fagaceae, Hydrangeaceae and Lamiaceae. Tropical Plant Pathology 42:239–249
Meeboon J, Takamatsu S (2017b) Notes on powdery mildews (Erysiphales) in Thailand IV. Erysiphe species on Malvaceae, Menispermaceae, Moraceae, Nyctaginaceae, Polygonaceae, Solanaceae and Urticaceae. Tropical Plant Pathology. https://doi.org/10.1007/s40858-017-0156-2
Meeboon J, Takamatsu S (2017c) New records of Erysiphe sect. Uncinula spp. (Erysiphales) from Thailand and E. liquidambaris var. acalycinae var. nov. Mycoscience 58:236–241
Meeboon J, Takamatsu S (2017d) First found of Erysiphe elevata on Eucalyptus camaldulensis and Phyllactinia lagerstroemiae sp. nov. on Lagerstroemia from Thailand. Mycoscience 58:253–260
Meeboon J, Hidayat I, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand I. Podosphaera sect. Sphaerotheca. Tropical Plant Pathology 6:142–174
Narayanaswami P, Ramakrishnan K (1967–1968) 1969 powdery mildews of Coimbatore, madras state. The Madras University Journal 37–38:84–99
Park MJ, Hong SH, Cho SE, Park JH, Shin HD (2015) First report of powdery mildew caused by Golovinomyces orontii on invasive weed Lactuca serriola in Korea. Plant Disease 99:889
Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, Romberg M, Samson RA, Seifert KA, Stone JK, Udayanga D, White JF (2016) Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 7:289–308
Scholler M, Schmidt A, Siahaan SAS, Takamatsu S, Braun U (2016) A taxonomic and phylogenetic study of the Golovinomyces biocellatus complex (Erysiphales, Ascomycota) using asexual state morphology and rDNA sequence data. Mycological Progress 15:56
Shin HD (2000) Erysiphaceae of Korea. National Inst. of Agric. Sci. and Technol, Suwon, Korea
Shin HD, La YJ (1993) Morphology of edge lines of chained immature conidia on conidiophores in powdery mildew fungi and their taxonomic significance. Mycotaxon 66:445–451
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer, Sunderland
Takamatsu S, Kano Y (2001) PCR primers useful for nucleotide sequencing of rDNA of the powdery mildew fungi. Mycoscience 42:135–139
Takamatsu S, Matsuda S, Grigaliunaite B (2013) Comprehensive phylogenetic analysis of the genus Golovinomyces (Ascomycota: Erysiphales) reveals close evolutionary relationships with its host plants. Mycologia 105:1135–1152
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Vági P, Kovács GM, Kiss L (2007) Host range expansion in a powdery mildew fungus Golovinomyces sp. infecting Arabidopsis thaliana: Torenia fournieri as a new host. European Journal of Plant Pathology 117:89–93
Walker JB, Sytsma KJ, Treutlein J, Wink M (2004) Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. American Journal of Botany 91:1115–1125
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a mediumfor simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506–513
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Acknowledgements
This work was financially supported in part by a Grant-in-Aid for Scientific Research (No. 16K07613 and 16F16097) from the Japan Society for the Promotion of Science to ST; and The JSPS postdoctoral fellowship to JM (P16097).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Meike Piepenbring
Rights and permissions
About this article
Cite this article
Meeboon, J., Kokaew, J. & Takamatsu, S. Notes on powdery mildews (Erysiphales) in Thailand V. Golovinomyces . Trop. plant pathol. 43, 202–217 (2018). https://doi.org/10.1007/s40858-017-0201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-017-0201-1