Abstract
Purpose
The prostate-specific antigen (PSA) density (PSAD) in prostate cancer (PCa) detection has limited applicability and is probably caused by moderate accuracy. The purpose of this study was to create a machine learning (ML) PSAD model that incorporates PSAD predictors for forecasting clinically significant (cs) prostate cancer (PCa) probability and compare its performance to that of the traditional PSAD.
Methods
PSA and prostate volume (PV) were retrieved from the 725 patients that were subjected to prostate biopsy. After resampling and splitting data, we used the training set to create seven ML algorithms. We chose the RF model that was the most accurate. The area under the curve (AUC) accuracy, precision, sensitivity, and specificity of PSAD and RF PSAD diagnostic performance were compared. Additionally, the ML model’s explainability and its website placement were performed.
Results
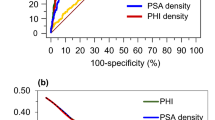
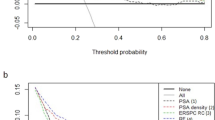
csPCa was found in 140 males (19.3%). The proposed novel model exhibited much higher evolution metrics than PSAD. AUC for the PSAD and RF PSAD were 0.757 and 0.942, respectively. The reliability diagram indicates that the RF model fits the data well. For the RF model, the decision curve analysis revealed a net benefit of more than 5%, and 40% subjects could avoid unnecessary biopsy. PV was the more important determinant for csPCa. PSA and PV had non-monotonic relationships and a lot of turbulence.
Conclusion
The RF PSAD model demonstrated strong discrimination and clinical value, which could aid urologists in determining whether a prostate biopsy is required.




Similar content being viewed by others
References
Rawla, P. (2019). Epidemiology of prostate Cancer. World Journal of Oncology, 10(2), 63–89.
Benson, M. C., Whang, I. S., Pantuck, A., et al. (1992). Prostate specific antigen density: A means of distinguishing benign prostatic hypertrophy and prostate cancer. Journal of Urology, 147(3 Pt 2), 815–816.
Yusim, I., Krenawi, M., Mazor, F., et al. (2020). The use of prostate specific antigen density to predict clinically significant prostate cancer. Scientific Reports, 10(1), 20015.
Nordström, T., Akre, O., Aly, M., et al. (2018). Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer and Prostatic Diseases, 21(1), 57–63.
Jue, J. S., Barboza, M. P., Prakash, N. S., et al. (2017). Re-examining prostate-specific Antigen (PSA) density: Defining the optimal PSA Range and patients for using PSA Density to predict prostate Cancer using extended Template Biopsy. Urology, 105, 123–128.
Danacioglu, Y. O., Keser, F., Efiloğlu, Ö., et al. (2021). The efficiency of prostate-specific antigen density measurement using three different methods on the prediction of biochemical recurrence. The Aging Male : The Official Journal of the International Society for the Study of the Aging Male, 24(1), 15–23.
Peng, C., Zhang, J., & Hou, J. (2019). Performance characteristics of prostate-specific antigen density and biopsy primary gleason score to predict biochemical failure in patients with intermediate prostate cancer who underwent radical prostatectomy. Cancer Management and Research, 11, 1133–1139.
Brassetti, A., Lombardo, R., Emiliozzi, P., et al. (2018). Prostate-specific Antigen density is a good predictor of upstaging and upgrading, according to the New Grading System: The Keys we are seeking May be already in our Pocket. Urology, 111, 129–135.
Ediz, C., Akan, S., & Temel, M. C. (2020). The importance of PSA-density in active surveillance for prostate cancer. Archivio Italiano Di Urologia E Andrologia. https://doi.org/10.4081/aiua.2020.2.136
Washington, S. L., 3rd., Baskin, A. S., Ameli, N., et al. (2020). MRI-based prostate-specific antigen density predicts gleason score upgrade in an active surveillance cohort. American Journal of Roentgenology, 214, 574–578.
Press, B. H., Khajir, G., Ghabili, K., et al. (2021). Utility of PSA Density in Predicting upgraded gleason score in men on active Surveillance with negative MRI. Urology, 155, 96–100.
Wang, C., Wang, Y. Y., Wang, S. Y., et al. (2021). Peripheral zone PSA density: A predominant variable to improve prostate cancer detection efficiency in men with PSA higher than 4 ng ml– 1. Asian Journal of Andrology, 23, 415–420.
Lee, J., Yang, S. W., Jin, L., et al. (2021). Is PSA density of the peripheral zone as a useful predictor for prostate cancer in patients with gray zone PSA levels? Bmc Cancer, 21(1), 472.
Castro, H. A. S., Iared, W., Santos, J. E. M., et al. (2018). Impact of PSA density of transition zone as a potential parameter in reducing the number of unnecessary prostate biopsies in patients with psa levels between 2.6 and 10.0 ng/mL. International Braz J Urol : Official Journal of the Brazilian Society of Urology, 44, 709–716.
Chang, T. H., Lin, W. R., Tsai, W. K., et al. (2020). Zonal adjusted PSA density improves prostate cancer detection rates compared with PSA in Taiwanese males with PSA < 20 ng/ml. Bmc Urology, 20(1), 151.
Schneider, A. F., Stocker, D., Hötker, A. M., et al. (2019). Comparison of PSA-density of the transition zone and whole gland for risk stratification of men with suspected prostate cancer: A retrospective MRI-cohort study. European Journal of Radiology, 120, 108660.
Omri, N., Kamil, M., Alexander, K., et al. (2020). Association between PSA density and pathologically significant prostate cancer: The impact of prostate volume. Prostate, 80(16), 1444–1449.
Feng, Z. J., Xue, C., Wen, J. M., et al. (2017). PSAD test in the diagnosis of prostate cancer: a meta-analysis. Clinical Laboratory, 63(1), 147–155.
Frisbie, J. W., Van Besien, A. J., Lee, A., et al. (2022). PSA density is complementary to prostate MP-MRI PI-RADS scoring system for risk stratification of clinically significant prostate cancer. Prostate Cancer and Prostatic Diseases. https://doi.org/10.1038/s41391-022-00549-y
Morote, J., Borque-Fernando, A., Triquell, M., et al. (2022). Comparative analysis of PSA Density and an MRI-Based predictive model to improve the selection of candidates for prostate biopsy. Cancers (Basel), 14(10), 2374.
Chiu, P. K., Shen, X., Wang, G., et al. (2021). Enhancement of prostate cancer diagnosis by machine learning techniques: An algorithm development and validation study. Prostate Cancer and Prostatic Diseases, 25(4), 672–676.
Gentile, F., Ferro, M., Della Ventura, B., et al. (2021). Optimized identification of high-grade prostate cancer by combining different PSA molecular forms and PSA density in a deep learning model. Diagnostics (Basel), 11(2), 335.
Epstein, J. I., Egevad, L., Amin, M. B., Grading Committee. (2016). The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. The American Journal of Surgical Pathology, 40(2), 244.
September H2O.ai, Distributed Random Forest (DRF). (2021). https://docs.h2o.ai/h2o/latest-stable/h2o-docs/data-science/drf.html H2O version 3.34.0.1
Xiao, L. H., Chen, P. R., Gou, Z. P., et al. (2017). Prostate cancer prediction using the random forest algorithm that takes into account transrectal ultrasound findings, age, and serum levels of prostate-specific antigen. Asian Journal of Andrology, 19(5), 586–590.
Wang, G., Teoh, J. Y., & Choi, K. S. (2018). Diagnosis of prostate cancer in a Chinese population by using machine learning methods. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 1-4). IEEE.
Roobol, M. J., vanVugt, H. A., Loeb, S., et al. (2012). Prediction of prostate cancer risk: The role of prostate volume and digital rectal examination in the ERSPC risk calculators. European Urology, 61(3), 577–583.
Yamashiro, J. R., & de Riese, W. T. W. (2021). Any correlation between prostate volume and incidence of prostate Cancer: A review of reported data for the last thirty years. Research and Reports in Urology, 13, 749–757.
Moolupuri, A., Camacho, J., & de Riese, W. T. (2021). Association between prostate size and the incidence of prostate cancer: A meta-analysis and review for urologists and clinicians. International Urology and Nephrology, 53(10), 1955–1961.
Lorenzo, G., Hughes, T. J. R., Dominguez-Frojan, P., et al. (2019). Computer simulations suggest that prostate enlargement due to benign prostatic hyperplasia mechanically impedes prostate cancer growth. Proceedings of the National Academy of Sciences, 116(4), 1152–1161.
Radtke, J. P., Wiesenfarth, M., Kesch, C., et al. (2017). Combined clinical parameters and multiparametric magnetic resonance imaging for Advanced Risk modeling of prostate Cancer-patient-tailored risk stratification can reduce unnecessary biopsies. European Urology, 72(6), 888–896.
Truong, M., Wang, B., Gordetsky, J. B., et al. (2018). Multi-institutional nomogram predicting benign prostate pathology on magnetic resonance/ultrasound fusion biopsy in men with a prior negative 12-core systematic biopsy. Cancer, 124(2), 278–285.
Alberts, A. R., Roobol, M. J., Verbeek, J. F. M., et al. (2019). Prediction of high-grade prostate Cancer following multiparametric magnetic resonance imaging: Improving the Rotterdam European Randomized Study of screening for prostate Cancer risk calculators. European Urology, 75(2), 310–318.
Charilaou, P., & Battat, R. (2022). Machine learning models and over-fitting considerations. World Journal of Gastroenterology, 28(5), 605–607.
Vickers, A. J., Van Calster, B., & Steyerberg, E. W. (2016). Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. Bmj, 352, i6.
Dekalo, S., Savin, Z., Schreter, E., et al. (2021). Novel ultrasound-based volume estimation of prostatic benign enlargement to improve decision-making on surgical approach. Therapeutic Advances in Urology, 13, 1756287221993301.
Acknowledgements
The authors were financially supported through a research grant N0175014, N175007and III 41007of the Ministry of Science and Technological Development of Serbia and Grants OI174028 from the City of Kragujevac. The authors thank the Ministry for this support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by [MS] and [BM]. The first draft of the manuscript was written by [MS]. Supervision was done by [SJ]. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stojadinovic, M., Milicevic, B. & Jankovic, S. Enhanced PSA Density Prediction Accuracy When Based on Machine Learning. J. Med. Biol. Eng. 43, 249–257 (2023). https://doi.org/10.1007/s40846-023-00793-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-023-00793-0