Abstract
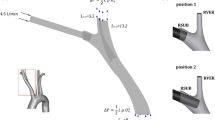
Cerebral hypoperfusion that may occur during linear cardiopulmonary bypass (CPB) is often responsible for mnemonic problems, loss of neural functions, or coma. Pulsed CPB might provide better organ protection, especially to the brain. The present work numerically investigates how the CPB cannula orientation influences blood flow in aortic and epiaortic vessels during pulsed CPB, realized using the intra-aortic balloon pump (IABP). The computational fluid dynamics (CFD) model consisted of a three-dimensional (3D) patient-specific aorta with three epiaortic vessels, a 40-cm3 intra-aortic balloon, and a 24 Fr arterial cannula in the traditional position and orientation (tilt angle of 45°, case I) and with a greater inclination (tilt angle of 60°, case II). Comparative multi-scale studies, realized by coupling a 3D CFD analysis and a lumped-parameters model, were carried out to establish the hemodynamic modifications due to changes in the cannula orientation. A comparison between the two cases revealed that when the cannula flow was directed to the epiaortic vessels (case I), the mean flows of the left subclavian artery and the thoracic aorta increased by about 2.08 and 7.50 %, respectively. When the flow cannula collided with the aortic arch concavity (case II), this area presented high wall shear stress (WSS) values and a greater amount of blood occurred in the ascending aorta, where it stagnated, although the mean flows of the innominate and left common carotid arteries increased by about 6.25 and 3.13 %, respectively. Pulsed CPB may reduce brain damage during heart surgery, so the evaluation of epiaortic flow during the extracorporeal circulation with various arterial cannula orientations is useful for the analysis of hemodynamic modifications.






Similar content being viewed by others
References
Machine, D., & Allsager, C. (2006). Principles of cardiopulmonary bypass. Continuing Education in Anaesthesia, Critical Care & Pain, 6, 176–181.
De Bartolo, C., Nigro, A., Fragomeni, G., Colacino, F. M., Wang, D., Jones, C. C., & Zwischenberger, J. (2011). Numerical and experimental flow analysis of the Wang-Zwische Double-Lumen cannula. ASAIO Journal, 57, 318–327.
Mulholland, J. W., Shelton, J. C., & Luo, X. Y. (2005). Blood flow and damage by the roller pumps during cardiopulmonary bypass. Journal of Fluids and Structures, 20, 129–140.
Westerhof, N., Elzinga, G., & Sipkema, P. (1971). An artificial arterial system for pumping hearts. Journal of Applied Physiology, 31, 776–781.
Hornick, P., & Taylor, K. M. (1997). Pulsatile and non-pulsatile perfusion: The continuing controversy. Journal of cardiothoracic and vascular anesthesia, 11, 310–315.
Voss, B., Krane, M., Jung, C., Brockmann, G., Braun, S., Günther, T., et al. (2010). Cardiopulmonary bypass with physiological flow and pressure curves: Pulse is unnecessary. European Journal of Cardio-Thoracic Surgery, 37, 223–232.
Grossi, E. A., Connolly, M. W., Krieger, K. H., Nathan, I. M., Hunter, C. E., Colvin, S. B., et al. (1985). Quantification of pulsatile flow during cardiopulmonary bypass to permit direct comparison of the effectiveness of various types of ‘pulsatile’ and ‘nonpulsatile’ flow. Surgery, 98, 547–554.
Taylor, K. M., Devlin, B. J., Mittra, S. M., Gillan, J. G., Brannan, J. J., & McKenna, J. M. (1980). Assessment of cerebral damage during open heart surgery. A new experimental model. Scandinavian Journal of Thoracic and Cardiovascular Surgery, 14, 197–203.
Taylor, K. M. (1986). Pulsatile perfusion. In K. M. Taylor (Ed.), Cardiopulmonary bypass-principles and management. London: Chapman and Hall.
Jacobs, L. A., Klopp, E. H., Seamone, W., Topaz, S. R., & Gott, V. L. (1969). Improved organ function during cardiac bypass with a roller pump modified to deliver pulsatile flow. Journal of Thoracic and Cardiovascular Surgery, 58, 703–712.
Dunn, J., Kirsh, M. M., Harness, J., Carroll, M., Straker, J., & Sloan, H. (1974). Hemodynamic, metabolic and hematologic effects of pulsatile cardiopulmonary bypass. Journal of Thoracic and Cardiovascular Surgery, 68, 138–147.
Simpson, J. C. (1981). Cerebral perfusion during cardiac surgery using cardiac bypass. In D. B. Longmore (Ed.), Towards safer cardiac surgery. Lancaster: MTP.
Kono, M., Orita, H., Shimanuki, T., Fukasawa, M., Inui, K., & Wasio, M. (1990). A clinical study of cerebral perfusion during pulsatile and non-pulsatile cardiopulmonary bypass. Nippon Geka Gakkai Zasshi, 91, 1016–1022.
Dabrowski, W., Rzecki, Z., Pilat, J., & Czajkowski, M. (2012). Brain damage in cardiac surgery patients. Current Opinion in Pharmacology, 12, 1–6.
Onorati, F., Presta, P., Fuiano, G., Mastroroberto, P., Comi, N., Pezzo, F., et al. (2007). A randomized trial of pulsatile perfusion using intra-aortic balloon pump versus non-pulsatile perfusion on short-term changes in kidney function during cardiopulmonary bypass during myocardial reperfusion. American Journal of Kidney Diseases, 50, 229–238.
Onorati, F., Cristodoro, L., Bilotta, M., Impiombato, B., Pezzo, F., Mastroroberto, P., et al. (2006). Intra-aortic balloon pumping during cardioplegic arrest preserves lung function in patients with chronic obstructive pulmonary disease. Annals of Thoracic Surgery, 82, 35–43.
Onorati, F., Esposito, A., Comi, M. C., Impiombato, B., Cristodoro, L., Mastroroberto, P., & Renzulli, A. (2008). Intra-aortic balloon pump-induced pulsatile flow reduces coagulative and fibrinolytic response to cardiopulmonary bypass. Artificial Organs, 32, 433–441.
Gramigna, V., Caruso, M. V., Rossi, M., Serraino, G. F., Renzulli, A., & Fragomeni, G. (2014). A numerical analysis of the aortic blood flow pattern during pulsed cardiopulmonary bypass. Computer Methods in Biomechanics and Biomedical Engineering, 25, 1–8.
Kaufmann, T. A., Hormes, M., Laumen, M., Timms, D. L., Linde, T., Schmitz-Rode, T., et al. (2009). The impact of aortic/subclavian outflow cannulation for cardiopulmonary bypass and cardiac support: A computational fluid dynamics study. Artificial Organs, 33, 727–732.
Kaufmann, T. A., Hormes, M., Laumen, M., Timms, D. L., Schmitz-Rode, T., Moritz, A., et al. (2009). Flow distribution during cardiopulmonary bypass in dependency on the outflow cannula positioning. Artificial Organs, 33, 988–992.
Jegger, D., Sundaram, S., Shah, K., Mallabiabarrena, I., Mucciolo, G., & von Segesser, L. K. (2007). Using computational fluid dynamics to evaluate a novel venous cannula (Smart canula) for use in cardiopulmonary bypass operating procedures. Perfusion, 22, 257–265.
Menon, P. G., Antaki, J. F., Undar, A., & Pekkan, K. (2013). Aortic outflow cannula tip design and orientation impacts cerebral perfusion during pediatric cardiopulmonary bypass procedure. Annals of Biomedical Engineering, 41, 2588–2602.
Tokuda, Y., Song, M. H., Ueda, Y., Usui, A., Akita, T., Yoneyama, S., & Maruyama, S. (2008). Three-dimensional numerical simulation of blood flow in the aortic arch during cardiopulmonary bypass. European Journal of Cardio-Thoracic Surgery, 33, 164–167.
Vignon-Clementel, I. E., Figueroa, C. A., Jansen, K. E., & Taylor, C. A. (2006). Outflow boundary conditions for three dimensional finite element modeling of blood flow and pressure in arteries. Computer Methods in Applied Mechanics and Engineering, 195, 3776–3796.
Vignon-Clementel, I. E. (2006). A coupled multidomain method for computational modeling of blood flow. Ph.D. dissertation, Stanford University, Mechanical Engineering Department.
Serraino, G. F., Marsico, R., Musolino, G., Ventura, V., Gulletta, E., Sante, P., & Renzulli, A. (2011). Pulsatile cardiopulmonary bypass with intra-aortic balloon pump improves organ function and reduces endothelial activation. Circulation Journal, 76, 1121–1129.
Kern, M. J., Aguirre, F. V., Caracciolo, E. A., Bach, R. G., Donohue, T. J., Lasorda, D., et al. (1999). Hemodynamic effects of new intra-aortic balloon counterpulsation timing methods in patients: A multicenter evaluation. American Heart Journal, 137, 1129–1136.
Klopman, M. A., Chen, E. P., & Sniecinski, R. M. (2011). Positioning an intraaortic balloon pump using intraoperative transesophageal echocardiogram guidance. Anesthesia and Analgesia, 113, 40–43.
Formaggia, L., Perktold, K., & Quarteroni, A. (2009). Basic mathematical models and motivations. In A. Quarteroni, L. Formaggia, & A. Veneziani (Eds.), Cardiovascular mathematics: Modeling and simulation of the circulatory system (pp. 47–75). Milan: Spinger.
Figueroa, C. A., Vignon-Clementel, I. E., Jansen, K. E., Hughes, T. J. R., & Taylor, C. A. (2006). A coupled momentum method for modeling blood flow in three dimensional deformable arteries. Computer Methods in Applied Mechanics and Engineering, 195, 5685–5706.
Stalder, A. F., Russe, M. F., Frydrychowicz, A., Bock, J., Henning, J., & Markl, M. (2008). Quantitative 2D and 3D phase contrast MRI: Optimized analysis of blood flow and vessel wall parameters. Magnetic Resonance in Medicine, 60, 1218–1231.
Malek, A. M., Alper, S. L., & Izumo, S. (1999). Hemodynamic shear stress and its role in atherosclerosis. JAMA, 282, 2035–2042.
Siegemund, M., & Steiner, L. A. (2013). Brain perfusion and autoregulation in systemic critical illness. In M. Siegemund & L. A. Steiner (Eds.), Brain disorders in critical illness, mechanism, diagnosis, and treatment (pp. 129–138). Cambridge: Cambridge University Press.
Kaufmann, T. A., Schmitz-Rode, T., & Steinseifer, U. (2012). Implementation of cerebral autoregulation into computational fluid dynamics studies of cardiopulmonary bypass. Artificial Organs, 36(8), 754–758.
Hammon, J. W. (2008). Extracorporeal circulation: The response of humoral and cellular elements of blood to extracorporeal circulation. In L. H. Cohn (Ed.), Cardiac surgery in the adult (pp. 370–389). New York: McGraw-Hill.
Mukherjee, N. D., Beran, A. V., Hirai, J., Wakabayashi, A., Sperling, D. R., Taylor, W. F., & Connolly, J. E. (1973). In vivo determination of renal tissue oxygenation during pulsatile and non-pulsatile left heart bypass. Annals of Thoracic Surgery, 15, 334–363.
Pappas, G., Winter, S. D., Kopriva, C. J., & Steele, P. P. (1975). Improvement of myocardial and other vital organ functions and metabolism with a simple method of pulsatile flow during clinical cardiopulmonary bypass. Surgery, 77, 34–44.
Lu, Y. H., Chen, C. Y., Menon, P. G., Liu, K. T., & Lin, H. H. (2014). Hemodynamic effects of endoleak formation in abdominal aortic aneurysm patients with Stent-Graft implants. Journal of Medical and Biological Engineering, 34(6), 554–558.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caruso, M.V., Gramigna, V., Serraino, G.F. et al. Influence of Aortic Outflow Cannula Orientation on Epiaortic Flow Pattern During Pulsed Cardiopulmonary Bypass. J. Med. Biol. Eng. 35, 455–463 (2015). https://doi.org/10.1007/s40846-015-0053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-015-0053-4