Abstract
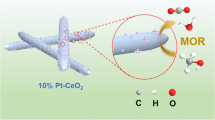
Modulating the electronic structure of Pt is an effective strategy for enhancing the activity and durability of Pt-based electrocatalysts. Herein, we reported a type of intermetallic CeO2/PtBi nanoplates (NPs), which possessed enhanced activity and durability for methanol electro-oxidation reaction (MOR) through strong p-d hybridization between the Pt and Bi. The surface-deposited CeO2 can further optimize the electronic structure of Pt, while providing more hydroxyl adsorption sites. Specifically, the CeO2/PtBi NPs exhibited excellent mass activity for MOR in both acidic and alkaline environments, which were 1.62 and 7.65 times higher than those of commercial Pt/C, respectively. After 1000 durability tests in acidic and alkaline environments, the activities of CeO2/PtBi NPs only decreased by 20.1% and 39.8%, respectively, while the activities of commercial Pt/C decreased by 55.4% and 78.5%, respectively. The excellent activity and durability can be attributed to the modulation of the electronic structure through p-d orbital hybridization between Pt, Bi and the surface-deposited CeO2. This study provides new insights into the electronic structure regulation of Pt-based electrocatalysts.

摘要
调控铂(Pt)电子结构是增强Pt基电催化剂活性和耐久性的关键 策略. 本研究开发了CeO2/PtBi金属间纳米板(NPs), 这种结构通过Pt和 Bi之间的强p-d轨道杂化, 显著提高了催化剂在甲醇电氧化反应(MOR) 中的活性和稳定性. CeO2的表面沉积不仅进一步优化了Pt的电子结构, 还提供了额外的羟基吸附位点. 特别是, 在酸性和碱性环境下, CeO2/PtBi NPs展现出超越商业Pt/C的甲醇电氧化质量活性, 分别提高了1.62 和7.65倍. 在经历了1000次耐久性测试后, CeO2/PtBi NPs在酸碱环境下 的活性仅分别下降了20.1%和39.8%, 相比之下, 商业Pt/C的活性在相同 条件下分别下降了55.4%和78.5%. CeO2/PtBi NPs的这一卓越性能得益 于Pt与Bi之间的p-d轨道杂化以及CeO2表面工程对催化剂电子结构的 有效调节. 本研究为Pt基电催化剂设计提供了新的电子结构调控策略 和深入见解.
Similar content being viewed by others
References
Song C. Fuel processing for low-temperature and high-temperature fuel cells Challenges, and opportunities for sustainable development in the 21st century. Catal Today, 2002, 77: 17–49
Aricò AS, Srinivasan S, Antonucci V. DMFCs: From fundamental aspects to technology development. Fuel Cells, 2001, 1: 133–161
Dillon R, Srinivasan S, Aricò AS, et al. International activities in DMFC R&D: Status of technologies and potential applications. J Power Sources, 2004, 127: 112–126
Stamenkovic VR, Mun BS, Arenz M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat Mater, 2007, 6: 241–247
Malyshkina N, Niemeier D. Future sustainability forecasting by exchange markets: basic theory and an application. Environ Sci Technol, 2010, 44: 9134–9142
Cao S, Tao FF, Tang Y, et al. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem Soc Rev, 2016, 45: 4747–4765
Qi X, Ye N, Zhang R, et al. Boosting the activity and CO tolerance for methanol oxidation reaction in alkaline media by constructing the Pt-TMNs electrocatalysts. Fuel, 2023, 350: 128773
Gasteiger HA, Kocha SS, Sompalli B, et al. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl Catal B-Environ, 2005, 56: 9–35
Eppinger J, Huang KW. Formic acid as a hydrogen energy carrier. ACS Energy Lett, 2017, 2: 188–195
Xu X, Fang C, Bi T, et al. Dodecahedral Au/Pt nanobowls as robust plasmonic electrocatalysts for methanol oxidation under visible-light illumination. Chem Eur J, 2020, 26: 10787–10794
Huang H, Wang X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J Mater Chem A, 2014, 2: 6266–6291
Kang Y, Pyo JB, Ye X, et al. Synthesis, shape control, and methanol electro-oxidation properties of Pt-Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano, 2012, 6: 5642–5647
Zhu J, Yang Y, Chen L, et al. Copper-induced formation of structurally ordered Pt-Fe-Cu ternary intermetallic electrocatalysts with tunable phase structure and improved stability. Chem Mater, 2018, 30: 5987–5995
Lin Y, Cui X, Yen CH, et al. PtRu/carbon nanotube nanocomposite synthesized in supercritical fluid: A novel electrocatalyst for direct methanol fuel cells. Langmuir, 2005, 21: 11474–11479
Zhao X, Yin M, Ma L, et al. Recent advances in catalysts for direct methanol fuel cells. Energy Environ Sci, 2011, 4: 2736–2753
Nan H, Su YQ, Tang C, et al. Engineering the electronic and strained interface for high activity of PdMcore@Ptmonolayer electrocatalysts for oxygen reduction reaction. Sci Bull, 2020, 65: 1396–1404
Tian XL, Wang L, Deng P, et al. Research advances in unsupported Pt-based catalysts for electrochemical methanol oxidation. J Energy Chem, 2017, 26: 1067–1076
Wu Z, Dang D, Tian X. Designing robust support for Pt alloy nanoframes with durable oxygen reduction reaction activity. ACS Appl Mater Interfaces, 2019, 11: 9117–9124
Liao W, Zhou S, Wang Z, et al. Composition-controlled effects of Pb content in PtPbRu trimetallic nanoparticles on the electrocatalytic oxidation performance of methanol. Fuel, 2022, 308: 122073
Chen C, Sun M, Wang K, et al. Dual-metal single-atomic catalyst: The challenge in synthesis, characterization, and mechanistic investigation for electrocatalysis. SmartMat, 2022, 3: 533–564
Yang N, Chen D, Cui P, et al. Heterogeneous nanocomposites consisting of Pt3Co alloy particles and CoP2 nanorods towards high-efficiency methanol electro-oxidation. SmartMat, 2021, 2: 234–245
Cui C, Gan L, Heggen M, et al. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat Mater, 2013, 12: 765–771
Beermann V, Gocyla M, Willinger E, et al. Rh-doped Pt-Ni octahedral nanoparticles: Understanding the correlation between elemental distribution, oxygen reduction reaction, and shape stability. Nano Lett, 2016, 16: 1719–1725
Lin R, Cai X, Zeng H, et al. Stability of high-performance Pt-based catalysts for oxygen reduction reactions. Adv Mater, 2018, 30: 1705332
Gao L, Li X, Yao Z, et al. Unconventional p-d hybridization interaction in PtGa ultrathin nanowires boosts oxygen reduction electrocatalysis. J Am Chem Soc, 2019, 141: 18083–18090
Ai X, Zou X, Chen H, et al. Transition-metal-boron intermetallics with strong interatomic d-sp orbital hybridization for high-performance electrocatalysis. Angew Chem Int Ed, 2020, 59: 3961–3965
Li J, Wang C, Shang H, et al. Metal-modified PtTe2 nanorods: Surface reconstruction for efficient methanol oxidation electrocatalysis. Chem Eng J, 2021, 424: 130319
Guo J, Gao L, Tan X, et al. Template-directed rapid synthesis of Pd-based ultrathin porous intermetallic nanosheets for efficient oxygen reduction. Angew Chem Int Ed, 2021, 60: 10942–10949
Wang Y, Wang D, Li Y. A fundamental comprehension and recent progress in advanced Pt-based ORR nanocatalysts. SmartMat, 2021, 2: 56–75
Li X, Fang Y, Wang J, et al. Ordered clustering of single atomic Te vacancies in atomically thin PtTe2 promotes hydrogen evolution catalysis. Nat Commun, 2021, 12: 2351
Liang W, Wang Y, Zhao L, et al. 3D anisotropic Au@Pt-Pd hemispherical nanostructures as efficient electrocatalysts for methanol, ethanol, and formic acid oxidation reaction. Adv Mater, 2021, 33: 2100713
Qin Y, Zhang W, Wang F, et al. Extraordinary p-d hybridization interaction in heterostructural Pd-PdSe nanosheets boosts C–C bond cleavage of ethylene glycol electrooxidation. Angew Chem Int Ed, 2022, 61: e202200899
Cao X, Tian Y, Ma J, et al. Strong p-d orbital hybridization on bismuth nanosheets for high performing CO2 electroreduction. Adv Mater, 2024, 36: 2309648
Chen W, Luo S, Sun M, et al. Hexagonal PtBi intermetallic inlaid with sub-monolayer Pb oxyhydroxide boosts methanol oxidation. Small, 2022, 18: 2107803
Li T, Li S, Liu Q, et al. Hollow Co3O4/CeO2 heterostructures in situ embedded in N-doped carbon nanofibers enable outstanding oxygen evolution. ACS Sustain Chem Eng, 2019, 7: 17950–17957
Qiu B, Wang C, Zhang N, et al. CeO2-induced interfacial Co2+ octahedral sites and oxygen vacancies for water oxidation. ACS Catal, 2019, 9: 6484–6490
Sivanantham A, Ganesan P, Shanmugam S. A synergistic effect of Co and CeO2 in nitrogen-doped carbon nanostructure for the enhanced oxygen electrode activity and stability. Appl Catal B-Environ, 2018, 237: 1148–1159
Zhang Y, Ye F, Li W-Z. Self-assembled two-dimensional NiO/CeO2 heterostructure rich in oxygen vacancies as efficient bifunctional electrocatalyst for alkaline hydrogen evolution and oxygen evolution. Chem Eur J, 2021, 27: 3766–3771
Chen S, Zheng Z, Li Q, et al. Boosting electrocatalytic water oxidation of NiFe layered double hydroxide via the synergy of 3d–4f electron interaction and citrate intercalation. J Mater Chem A, 2023, 11: 1944–1953
Lv L, Zha D, Ruan Y, et al. A Universal method to engineer metal oxide-metal-carbon interface for highly efficient oxygen reduction. ACS Nano, 2018, 12: 3042–3051
Dai T, Zhang X, Sun M, et al. Uncovering the promotion of CeO2/ CoS1.97 heterostructure with specific spatial architectures on oxygen evolution reaction. Adv Mater, 2021, 33: 2102593
Xu H, Cao J, Shan C, et al. MOF-derived hollow CoS decorated with CeOx nanoparticles for boosting oxygen evolution reaction electrocatalysis. Angew Chem Int Ed, 2018, 57: 8654–8658
Li T, Yin J, Sun D, et al. Manipulation of Mott-Schottky Ni/CeO2 heterojunctions into N-doped carbon nanofibers for high-efficiency electrochemical water splitting. Small, 2022, 18: 2106592
Gu DM, Chu YY, Wang ZB, et al. Methanol oxidation on Pt/CeO2-C electrocatalyst prepared by microwave-assisted ethylene glycol process. Appl Catal B-Environ, 2011, 102: 9–18
Yang F, Bao X, Li P, et al. Boosting hydrogen oxidation activity of Ni in alkaline media through oxygen-vacancy-rich CeO2/Ni heterostructures. Angew Chem Int Ed, 2019, 58: 14179–14183
Liu C, Zhang L, Sun L, et al. Enhanced electrocatalytic activity of PtCu bimetallic nanoparticles on CeO2/carbon nanotubes for methanol electro-oxidation. Int J Hydrogen Energy, 2020, 45: 8558–8567
Huang L, Zhang X, Wang Q, et al. Shape-control of Pt-Ru nanocrystals: Tuning surface structure for enhanced electrocatalytic methanol oxidation. J Am Chem Soc, 2018, 140: 1142–1147
Wang Y, Zheng M, Li Y, et al. Oxygen-bridged long-range dual sites boost ethanol electrooxidation by facilitating C–C bond cleavage. Nano Lett, 2023, 23: 8194–8202
Formo E, Peng Z, Lee E, et al. Direct oxidation of methanol on Pt nanostructures supported on electrospun nanofibers of anatase. J Phys Chem C, 2008, 112: 9970–9975
Xu X, Zhang X, Sun H, et al. Synthesis of Pt-Ni alloy nanocrystals with high-index facets and enhanced electrocatalytic properties. Angew Chem, 2014, 126: 12730–12735
Wu P, Zhang H, Qian Y, et al. Composition- and aspect-ratio-dependent electrocatalytic performances of one-dimensional aligned Pt–Ni nanostructures. J Phys Chem C, 2013, 117: 19091–19100
Gong M, Fu G, Chen Y, et al. Autocatalysis and selective oxidative etching induced synthesis of platinum-copper bimetallic alloy nanodendrites electrocatalysts. ACS Appl Mater Interfaces, 2014, 6: 7301–7308
Wang X, Liu Y, Ma XY, et al. The role of bismuth in suppressing the CO Poisoning in alkaline methanol electrooxidation: switching the reaction from the CO to formate pathway. Nano Lett, 2023, 23: 685–693
Li P, Bi J, Liu J, et al. p-d orbital hybridization induced by p-block metal-doped Cu promotes the formation of C2+ products in ampere-level CO2 electroreduction. J Am Chem Soc, 2023, 145: 4675–4682
Zhang G, Hui C, Yang Z, et al. Hydrogen-induced p-d orbital hybridization and tensile strain of PdGa single-atom alloy metallene boosts complete electrooxidation of ethanol. Appl Catal B-Environ, 2024, 342: 123377
Wang Y, Zheng M, Li Y, et al. p-d orbital hybridization induced by a monodispersed Ga site on a Pt3Mn nanocatalyst boosts ethanol electrooxidation. Angew Chem Int Ed, 2022, 61: e202115735
Huang L, Han Y, Zhang X, et al. One-step synthesis of ultrathin PtxPb nerve-like nanowires as robust catalysts for enhanced methanol electrooxidation. Nanoscale, 2017, 9: 201–207
Peng R, Zhang H, Guo Y, et al. The lanthanide doping effect on toluene catalytic oxidation over Pt/CeO2 catalyst. J Colloid Interface Sci, 2022, 614: 33–46
Acknowledgements
This work was supported by Shandong Excellent Young Scientists Fund Program (2022HWYQ-082) and the National Nature Science Foundation of China (22278174).
Author information
Authors and Affiliations
Contributions
Author contributions Zhang Y and Xue R conceived the idea. Zhang Y performed the experiments with support from Gao D. Lv Y performed the calculation of the theoretical model. Wang S, Chen G and Si F revised the manuscript and gave guidances. Zhang Y and Gao D wrote the paper and directed the project. All authors commented and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Yanao Zhang is a Master student at the University of Jinan. He is currently conducting his research under the supervision of associate professor Daowei Gao. His research primarily focuses on direct methanol fuel cells.
Daowei Gao is an associate professor at the School of Chemistry and Chemical Engineering, University of Jinan. He received his PhD degree from China University of Petroleum-Beijing, and undertook a postdoctoral research fellowship at Helmholtz-Zentrum Berlin für Materialien und Energie. His current researches focus mainly on physical chemistry of heterogeneous catalysis and proton exchange membrane fuel cells.
Supporting Information
40843_2023_2866_MOESM1_ESM.pdf
Synergistic Effects of p-d Orbital Hybridization and CeO2 Surface Engineering on PtBi Nanoplates for Methanol Electro-Oxidation
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, S., Si, F. et al. Synergistic effects of p-d orbital hybridization and CeO2 surface engineering on PtBi nanoplates for methanol electro-oxidation. Sci. China Mater. 67, 1975–1984 (2024). https://doi.org/10.1007/s40843-023-2866-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2866-y