Abstract
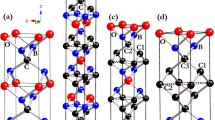
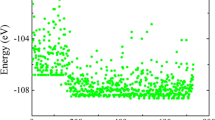
Boron-rich compounds with icosahedral-based structures possess rich, fascinating electronic and mechanical properties. Herein, the first comprehensive and systematic study of the crystal structures and properties of ternary B12CN and B13CN compounds with icosahedral structures has been performed by using particle swarm optimized structure prediction methods in combination with first-principles calculations. Compared with the widely studied variant structure of α-boron, the newly discovered Cmc21 structures are thermodynamically more stable for B12CN and B13CN. For structures with the same space group, B13CN possesses superior thermodynamic stability and mechanical properties than B12CN. Electronic structure calculations indicate that the boron-rich B-C-N system has abundant and tunable electronic properties, i.e., B13CN is a semiconductor, and B12CN possesses a hole-type conducting characteristic. The systematic study of structural ideal tensile strength indicates successive damage to icosahedra and successive bond breaks between icosahedra during tensile processes, leading to interesting deformation mechanisms, such as stress re-enhancement, structural multistep damage, and creep-like deformation.

摘要
以二十面体为基元的富硼化合物具有复杂多样的电子和机械性能. 在本工作中, 我们采用粒子群优化结构预测方法结合第一性原理计算, 首次对二十面体基元的三元B12CN和B13CN化合物的晶体结构和性质进行了全面系统的研究. 我们搜索得到了B13CN和B12CN化合物的新结构, 其空间群均为Cmc21, 与α-B的变体结构相比, 新结构具有更优异的热力学稳定性. B12CN的热力学稳定性和机械性能均稍逊于B13CN. 此外, B含量的微小差异造成了B12CN与B13CN两种三元化合物迥异的电学特性, 即B13CN具有半导体特性, 而B12CN具有空穴型导电特性. 此外, 在B12CN与B13CN系列新结构的拉伸过程中, 由于二十面体的连续破坏和二十面体之间的连续断键造成的应力再增强、 结构多级破坏和类蠕变变形等特殊变形机制也同样被揭示.
Similar content being viewed by others
References
Ma T, Li H, Zheng X, et al. Ultrastrong boron frameworks in ZrB12: A highway for electron conducting. Adv Mater, 2017, 29: 1604003
An Q, Goddard III WA. Nanotwins soften boron-rich boron carbide (B13C2). Appl Phys Lett, 2017, 110: 111902
Guo X, He J, Liu Z, et al. Bond ionicities and hardness of B13C2-like structured by ByX crystals (X = C, N, O, P, As). Phys Rev B, 2006, 73: 104115
Otani S, Nakagawa H, Nishi Y, et al. Floating zone growth and high temperature hardness of rare-earth hexaboride crystals: LaB6, CeB6, PrB6, NdB6, and SmB6. J Solid State Chem, 2000, 154: 238–241
Solozhenko VL, Bushlya V. Mechanical properties of superhard boron subnitride B13N2. J Superhard Mater, 2017, 39: 422–426
Akopov G, Yeung MT, Kaner RB. Rediscovering the crystal chemistry of borides. Adv Mater, 2017, 29: 1604506
Hoffmann RD, Fugmann A, Rodewald UC, et al. New intermetallic compounds Ln2Ni2Mg (Ln = Y, La-Nd, Sm, Gd-Tm) with Mo2FeB2 structure. Z anorg allg Chem, 2000, 626: 1733–1738
Zhang Y, Miller GJ, Fokwa BPT. Computational design of rare-earth-free magnets with the Ti3Co5B2-type structure. Chem Mater, 2017, 29: 2535–2541
Zhang Y, Sun SK, Guo WM, et al. Fabrication of textured (Hf0.2Zr0.2-Ta0.2Cr0.2Ti0.2)B2 high-entropy ceramics. J Eur Ceramic Soc, 2021, 41: 1015–1019
Liu J, Yang QQ, Zou J, et al. Strong high-entropy diboride ceramics with oxide impurities at 1800°C. Sci China Mater, 2023, 66: 2061–2070
Sergeeva AP, Piazza ZA, Romanescu C, et al. B22− and B23−: All-boron analogues of anthracene and phenanthrene. J Am Chem Soc, 2012, 134: 18065–18073
Popov IA, Piazza ZA, Li WL, et al. A combined photoelectron spectroscopy and ab initio study of the quasi-planar B24− cluster. J Chem Phys, 2013, 139: 144307
An Q, Goddard Iii WA. Microalloying boron carbide with silicon to achieve dramatically improved ductility. J Phys Chem Lett, 2014, 5: 4169–4174
Vast N, Sjakste J, Betranhandy E. Boron carbides from first principles. J Phys-Conf Ser, 2009, 176: 012002
Dong H, Oganov AR, Wang Q, et al. Prediction of a new ground state of superhard compound B6O at ambient conditions. Sci Rep, 2016, 6: 31288
Matkovich VI. Interstitial compounds of boron. J Am Chem Soc, 1961, 83: 1804–1806
Gao F, Hou L, He Y. Origin of superhardness in icosahedral B12 materials. J Phys Chem B, 2004, 108: 13069–13073
Bullett DW. Structure and bonding in crystalline boron and B12C3. J Phys C-Solid State Phys, 1982, 15: 415–426
Lazzari R, Vast N, Besson JM, et al. Atomic structure and vibrational properties of icosahedral B4C boron carbide. Phys Rev Lett, 1999, 83: 3230–3233
Xie KY, An Q, Toksoy MF, et al. Atomic-level understanding of “asymmetric twins” in boron carbide. Phys Rev Lett, 2015, 115: 175501
An Q, Goddard WA, Cheng T. Atomistic explanation of shear-induced amorphous band formation in boron carbide. Phys Rev Lett, 2014, 113: 095501
Domnich, V, Reynaud, S, Haber, RA, et al. Boron carbide: Structure, properties, and stability under stress. J Am Ceram Soc, 2011, 94: 3605–3628
Jay A, Vast N, Sjakste J, et al. Carbon-rich icosahedral boron carbide designed from first principles. Appl Phys Lett, 2014, 105: 031914
Solozhenko VL, Le Godec Y, Kurakevych OO. Solid-state synthesis of boron subnitride, B6N: Myth or reality? Comptes Rendus Chimie, 2006, 9: 1472–1475
Solozhenko VL, Kurakevych OO. New boron subnitride B13N2: HP-HT synthesis, structure and equation of state. J Phys-Conf Ser, 2008, 121: 062001
Çetin AÖ, Durandurdu M. Hard boron rich boron nitride nanoglasses. J Am Ceram Soc, 2018, 101: 1929–1939
Wang L, Sun R, Liu W, et al. Novel superhard boron-rich nitrides under pressure. Sci China Mater, 2020, 63: 2358–2364
Zhang X, Yu G, Cheng T, et al. New icosahedra-based B4N phases by particle swarm optimization. J Alloys Compd, 2021, 854: 157255
Bafekry A, Naseri M, Fadlallah MM, et al. A novel two-dimensional boron–carbon–nitride (BCN) monolayer: A first-principles insight. J Appl Phys, 2021, 130: 114301
Zhang Y, Sun H, Chen C. Superhard cubic BC2N compared to diamond. Phys Rev Lett, 2004, 93: 195504
Solozhenko VL, Turkevich VZ, Sato T. Phase stability of graphitelike BC4N up to 2100 K and 7 GPa. J Am Ceramic Soc, 1997, 80: 3229–3232
Zhu L, Ma M, Gao Q, et al. Prediction of a series of superhard BC4N structures. Diamond Relat Mater, 2022, 127: 109192
Bafekry A, Stampfl C. Band-gap control of graphenelike borocarbonitride g-BC6N bilayers by electrical gating. Phys Rev B, 2020, 102: 195411
Liang H, Li Q, Chen C. Atomistic mechanisms for contrasting stress–strain relations of B13CN and B13C2. J Phys Chem Lett, 2020, 11: 10454–10462
Wang Y, Lv J, Zhu L, et al. CALYPSO: A method for crystal structure prediction. Comput Phys Commun, 2012, 183: 2063–2070
Schmidt KM, Buettner AB, Graeve OA, et al. Interatomic pair potentials from DFT and molecular dynamics for Ca, Ba, and Sr hexaborides. J Mater Chem C, 2015, 3: 8649–8658
Blöchl PE. Projector augmented-wave method. Phys Rev B, 1994, 50: 17953–17979
Segall MD, Lindan PJD, Probert MJ, et al. First-principles simulation: Ideas, illustrations and the CASTEP code. J Phys-Condens Matter, 2002, 14: 2717–2744
Vanderbilt D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B, 1990, 41: 7892–7895
Hill R. The elastic behaviour of a crystalline aggregate. Proc Phys Soc A, 1952, 65: 349–354
Tian Y, Xu B, Zhao Z. Microscopic theory of hardness and design of novel superhard crystals. Int J Refractory Met Hard Mater, 2012, 33: 93–106
Krukau AV, Vydrov OA, Izmaylov AF, et al. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J Chem Phys, 2006, 125: 224106
Bachelet GB, Hamann DR, Schlüter M. Pseudopotentials that work: From H to Pu. Phys Rev B, 1982, 26: 4199–4228
Dubois SMM, Rignanese GM, Pardoen T, et al. Ideal strength of silicon: An ab initio study. Phys Rev B, 2006, 74: 235203
Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B, 1996, 54: 11169–11186
Wu ZJ, Zhao EJ, Xiang HP, et al. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys Rev B, 2007, 76: 054115
Longuet-Higgins HC, Roberts MDV. The electronic structure of an icosahedron of boron atoms. Proc R Soc Lond A, 1955, 230: 110–119
Amberger E, Stumpf W. Elemental boron. In: Buschbeck KC (ed.). Gmelin Handbook of Inorganic and Organometallic Chemistry. Berlin: Springer-Verlag, 1981. 1–112
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0703400), the National Natural Science Foundation of China (52073245, 52002118, 52202071, and 52202049), Macao Youth Scholars Program (AM2021015), and the Postdoctoral Science Preferential Funding of Hebei Province (B2022003021 and B2021005001).
Author information
Authors and Affiliations
Contributions
Author contributions The original idea was conceived by Zhu L, Ma M, Xin S, and He J. The manuscript was drafted by Zhu L, Ma M, Xiong M, Gao Q, Wu Y, Ying P, Wei X, Zhao Z, Xin S, He J, and Tian Y. All authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Li Zhu is a PhD candidate at the Center for High Pressure Science (CHiPS), State Key Laboratory of Metastable Materials Science and Technology, Yanshan University. Her research interest focuses on the theoretical design and experimental synthesis of novel metastable materials under high pressure and high temperature.
Mengdong Ma received his PhD degree from the CHiPS, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University in 2019. Then, he worked as a postdoctoral fellow at the South China University of Technology and Macau University of Science and Technology supported by Macao Young Scholars Program. His research interests include the design and synthesis of hard and superhard ceramics.
Shengwei Xin received his PhD degree in materials science from Yanshan University in 2011. Currently, he works as an associate professor at the Clean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University. His research focuses on the fabrication, microstructure, physical and mechanical properties of high-entropy and/or nanocrystalline metallic and ceramic materials.
Julong He is a professor at the CHiPS, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University. He obtained his PhD degree in engineering from Yanshan University in 2004. His research interests include the design and synthesis of superhard materials and novel metastable materials.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhu, L., Ma, M., Xiong, M. et al. First-principles study of novel icosahedral-based B12CN and B13CN structures. Sci. China Mater. 66, 4480–4488 (2023). https://doi.org/10.1007/s40843-023-2593-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2593-3