Abstract
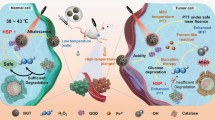
Dihydroartemisinin (DHA) has caught worldwide attention as an innovative antitumor drug. However, the inherent defects of DHA reduce its transport efficiency in the bloodstream, and insufficient iron content in the tumor cells further leads to poor therapeutic efficacy. Therefore, it is highly desirable to exploit a DHA/Fe-loaded nanoplatform which releases its payload responding to the tumor microenvironment (TME). Herein, we reported the smart nanodrugs CaCO3@DHA@Fe3+-tannic acid (TA)@polyethylene glycol (PEG) (CDFP) nanoparticles (NPs) that specifically release DHA and self-supply Fe2+ ions at the tumor site for synergistic cancer therapy. Fe3+-TA shell with good photothermal performance makes CDFP NPs suitable photothermal agents for photothermal therapy (PTT). Once CDFP NPs were internalized by tumor cells, TA, Fe3+, DHA, and Ca2+ could be released in the acidic TME. And TA can reduce Fe3+ to Fe2+, followed by Fe2+-DHA mediated chemodynamic reaction to yield reactive oxygen species (ROS). Meanwhile, intracellular overloaded Ca2+ ions lead to the mitochondrial dysfunction, which increases ROS production and further induces tumor cell apoptosis. Such CDFP NPs with PTT, Fe2+-DHA mediated ROS, and Ca2+ overloading show excellent antitumor effects in vitro and in vivo, certifying their great potential in further clinical application.

摘要
双氢青蒿素(DHA)作为一种新型的抗癌药物引起了全世界的广泛关注. 然而, DHA的固有缺陷降低了其在血液系统中的运输效率, 而肿瘤细胞中铁含量不足进一步导致疗效不佳. 因此, 构建一个可同时负载DHA和Fe3+并在肿瘤微环境中特异性释放出DHA/Fe的纳米药物具有重大意义. 本文报道了一种智能纳米药物CaCO3@DHA@Fe3+-TA@PEG (CDFP NPs)用于肿瘤协同治疗. Fe3+-TA壳层的包覆赋予CDFPNPs良好的光热性能. 此外, CDFP NPs在酸性的肿瘤微环境中可以快速降解并释放出TA, Fe3+, DHA和Ca2+. Fe3+与TA反应生成Fe2+, Fe2+与DHA相互作用生成高毒性的自由基. 此外, 在氧化应激状态下, Ca2+过载导致线粒体功能紊乱, 最终诱导肿瘤细胞死亡. 在光热治疗, 活性氧,Ca2+过载的协同作用下, CDFP NPs在体内外均表现出良好的治疗效果.
Similar content being viewed by others
References
Loibl S, Poortmans P, Morrow M, et al. Breast cancer. Lancet, 2021, 397: 1750–1769
Li M, Liu L, Xiao X, et al. The dynamic interactions between chemotherapy drugs and plasmid DNA investigated by atomic force microscopy. Sci China Mater, 2017, 60: 269–278
Jiang Y, Liu Y, Wang M, et al. siRNA-based carrier-free system for synergistic chemo/chemodynamic/RNAi therapy of drug-resistant tumors. ACS Appl Mater Interfaces, 2022, 14: 361–372
Shanmugam M, Kuthala N, Vankayala R, et al. Multifunctional CuO/Cu2O truncated nanocubes as trimodal image-guided near-infrared-III photothermal agents to combat multi-drug-resistant lung carcinoma. ACS Nano, 2021, 15: 14404–14418
Gao X, Wei M, Ma D, et al. Engineering of a hollow-structured Cu2−XS nano-homojunction platform for near infrared-triggered infected wound healing and cancer therapy. Adv Funct Mater, 2021, 31: 2106700
Sun Y, Hu H, Jing X, et al. Co-delivery of chemotherapeutic drugs and cell cycle regulatory agents using nanocarriers for cancer therapy. Sci China Mater, 2021, 64: 1827–1848
Li Y, Li M, Liu L, et al. Cell-specific metabolic reprogramming of tumors for bioactivatable ferroptosis therapy. ACS Nano, 2022, 16: 3965–3984
Bai S, Lu Z, Jiang Y, et al. Nanotransferrin-based programmable catalysis mediates three-pronged induction of oxidative stress to enhance cancer immunotherapy. ACS Nano, 2022, 16: 997–1012
Han W, Duan X, Ni K, et al. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials, 2022, 280: 121315
Zhu C, Wang Y, Li Z, et al. Metallopolysaccharide-based smart nanotheranostic for imaging-guided precise phototherapy and sequential enzyme-activated ferroptosis. Biomacromolecules, 2022, 23: 2007–2018
Wong YK, Xu C, Kalesh KA, et al. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med Res Rev, 2017, 37: 1492–1517
Li Y, Song Y, Zhang W, et al. MOF nanoparticles with encapsulated dihydroartemisinin as a controlled drug delivery system for enhanced cancer therapy and mechanism analysis. J Mater Chem B, 2020, 8: 7382–7389
Zhang X, Yang S, Wang Q, et al. Tailored theranostic nanoparticles cause efficient ferroptosis in head and neck squamous cell carcinoma through a reactive oxygen species “butterfly effect”. Chem Eng J, 2021, 423: 130083
Liu L, Wei Y, Zhai S, et al. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials, 2015, 62: 35–46
Cai L, Hu C, Liu S, et al. A covalent organic framework-based multifunctional therapeutic platform for enhanced photodynamic therapy via catalytic cascade reactions. Sci China Mater, 2021, 64: 488–497
Liang P, Tang H, Gu R, et al. A pH-responsive zinc(II) metalated porphyrin for enhanced photodynamic/photothermal combined cancer therapy. Sci China Mater, 2019, 62: 1199–1209
Zhu W, Chen M, Liu Y, et al. A dual factor activated metal-organic framework hybrid nanoplatform for photoacoustic imaging and synergetic photo-chemotherapy. Nanoscale, 2019, 11: 20630–20637
Fu LH, Wan Y, Qi C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater, 2021, 33: 2006892
Yang G, Xu L, Chao Y, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun, 2017, 8: 902
Dong Z, Feng L, Hao Y, et al. Synthesis of CaCO3-based nanomedicine for enhanced sonodynamic therapy via amplification of tumor oxidative stress. Chem, 2020, 6: 1391–1407
Mathesh M, Sun J, van der Sandt F, et al. Supramolecular nanomotors with “pH taxis” for active drug delivery in the tumor microenvironment. Nanoscale, 2020, 12: 22495–22501
Zheng P, Ding B, Jiang Z, et al. Ultrasound-augmented mitochondrial calcium ion overload by calcium nanomodulator to induce immunogenic cell death. Nano Lett, 2021, 21: 2088–2093
Zheng P, Ding B, Shi R, et al. A multichannel Ca2+ nanomodulator for multilevel mitochondrial destruction-mediated cancer therapy. Adv Mater, 2021, 33: 2007426
Zhou X, Sun WJ, Wang WM, et al. Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anti-Cancer Drugs, 2013, 24: 920–927
Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev, 2003, 83: 731–801
Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol, 2004, 44: 349–370
Naimin W, Sadrzadeh SMH. Enhancement of hemin-induced membrane damage by artemisinin. Biochem Pharmacol, 1994, 48: 737–741
Liu T, Zhang M, Liu W, et al. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano, 2018, 12: 3917–3927
Wei J, Wang G, Chen F, et al. Sol-gel synthesis of metal-phenolic coordination spheres and their derived carbon composites. Angew Chem Int Ed, 2018, 57: 9838–9843
Li J, Li X, Gong S, et al. Dual-mode avocado-like all-iron nanoplatform for enhanced T1/T2 MRI-guided cancer theranostic therapy. Nano Lett, 2020, 20: 4842–4849
Tian F, Wang S, Shi K, et al. Dual-depletion of intratumoral lactate and ATP with radicals generation for cascade metabolic-chemodynamic therapy. Adv Sci, 2021, 8: 2102595
Yan H, Ni H, Jia J, et al. Smart all-in-one thermometer-heater nanoprobe based on postsynthetical functionalization of a Eu(III)-metalorganic framework. Anal Chem, 2019, 91: 5225–5234
Liu T, Liu W, Zhang M, et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano, 2018, 12: 12181–12192
Liu J, Jin Y, Song Z, et al. Boosting tumor treatment by dredging the hurdles of chemodynamic therapy synergistic ion therapy. Chem Eng J, 2021, 411: 128440
Dong L, Wang C, Zhen W, et al. Biodegradable iron-coordinated hollow polydopamine nanospheres for dihydroartemisinin delivery and selectively enhanced therapy in tumor cells. J Mater Chem B, 2019, 7: 6172–6180
Wang D, Zhou J, Chen R, et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials, 2016, 107: 88–101
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2020YFA0712102), the National Natural Science Foundation of China (52022094, 22020102003, and 52072142), the Program of Science and Technology Development Plan of Jilin Province of China (20210101111JC and 20230508071RC), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2019232).
Author information
Authors and Affiliations
Contributions
Author contributions Wang Y designed the project. Wang Y, Wang D, Song S and Zhang H guided the project. Zhao Y performed the experiments with support from Cao Y, Zhou S, Liu Y, Niu R, Xu B, and Zhang S. All authors participated in general discussion of the paper.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Ying Zhao received her PhD degree from the University of Science and Technology of China in 2022. After that, she works as a lecturer at Henan University of Chinese Medicine. Her current research focuses on the design and preparation of photothermal nanomaterials for effective tumor therapy.
Yinghui Wang received her PhD degree in condensed matter physics from Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences (CAS) in 2011. Then, she worked as a postdoctoral researcher at the University of Amsterdam (Netherlands) and Changchun Institute of Applied Chemistry, CAS. Now, she is working as a professor at Changchun Institute of Applied Chemistry, CAS. Her research interests focus on the design and synthesis of multifunctional upconversion nanoparticles and their bioapplications.
Daguang Wang is a professor and chief physician, deputy director of the Department of Gastrocolorectal Surgery, First Hospital of Jilin University. He earned his MD degree from the Department of Gastrocolorectal Surgery, First Hospital of Jilin University. Afterwards, he was a visiting scholar at the Molecular Pathology Laboratory, Department of Pathology, Mount Sinai School of Medicine, USA in 2008. Then he worked as a visiting scholar at Minimally Invasive Surgery, Ohio State University College of Medicine, USA in 2015, and a clinical trainer at the Cancer Research Ariake Hospital, Japan in 2018. His research focuses on the minimally invasive surgical treatment of gastrointestinal tumors and bioinformatics, cell signaling in gastrointestinal tumors, functional nanomaterials in gastrointestinal tumors, and surgical nutrition support and metabolomics.
Hongjie Zhang received his BS degree (1978) from Peking University and MS degree (1985) from Changchun Institute of Applied Chemistry (CAS). Then, he worked as an assistant professor at the same institute from 1985–1989. He then studied at the University of Bordeaux I (France), where he received his PhD degree in 1993. He joined Changchun Institute of Applied Chemistry (CAS), as a professor in 1994. He was elected as an academician of the CAS in 2013, and a member of The World Academy of Science (TWAS) in 2015. His current research interests include the synthesis and application of lanthanide functional materials.
Supplementary information Supporting data are available in the online version of the paper.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, Y., Cao, Y. et al. Smart nanoplatform with programmed release of dihydroartemisinin and Fe2+ self-supply for synergistic cancer therapy. Sci. China Mater. 66, 4071–4078 (2023). https://doi.org/10.1007/s40843-023-2555-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2555-7