Abstract
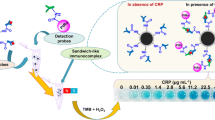
The sensitive detection of interferon alpha-2b (IFN-α2b) is crucial in treating viral infections and cancers. However, the natural enzymes used in the traditional enzyme-linked immunosorbent assays (ELISA) face the obstacles of high cost and low stability. Therefore, there is an urgent need to develop more cost-effective and stable alternatives to detect IFN-α2b. To address this issue, we synthesized poly-ethyleneimine (PEI)-modified magnetic nanoparticles (Fe3O4@PEI MNPs) that are highly stable, affordable and simple to prepare. These Fe3O4@PEI MNPs serve as a substitute for horseradish peroxidase in the ELISA-based detection of IFN-α2b, providing better sensitivity than chromatographic and traditional ELISA techniques. The peroxidase-like activity of Fe3O4@PEI MNPs can develop color, enabling the visualization of IFN-α2b. The proposed immunoassay has a linear increase with IFN-α2b concentrations ranging from 0.075 to 25 ng mL−1, with a low limit of detection of 0.055 ng mL−1. Based on the outstanding peroxidase-like activity of Fe3O4@PEI MNPs, this method has the potential to be used for the clinical detection of IFN-α2b and other protein biomarkers in disease diagnosis and treatments.

摘要
在病毒感染和癌症治疗中, 干扰素α-2b (IFN-α2b)的灵敏检测至关重要, 因此需要开发经济、 稳定的灵敏检测IFN-α2b的方法. 传统的酶联免疫吸附测定(ELISA)中使用的天然酶存在制备成本高和稳定性差等问题. 为了提高其灵敏度并降低成本, 我们合成了聚乙烯亚胺(PEI) 修饰的四氧化三铁磁性纳米粒子(Fe3O4@PEI MNPs). 在基于ELISA的IFN-α2b检测中, 这些磁性纳米粒子作为辣根过氧化物酶的替代品, 提供了比色谱和传统ELISA技术更高的灵敏度, 并且能够实现IFN-α2b的可视化检测. 该免疫分析方法的线性范围为0.075–25 ng mL−1, 检测限为0.055 ng mL−1. 基于Fe3O4@PEI MNPs优异的过氧化物酶活性, 该方法在用于检测IFN-α2b和其他蛋白质生物标志物监测方面具有临床应用潜力.
Similar content being viewed by others
References
Li Y, Rao C, Tao L, et al. Improved detection of variants in recombinant human interferon alpha-2a products by reverse-phase high-performance liquid chromatography on a core-shell stationary phase. J Pharm Biomed Anal, 2014, 88: 123–129
Ceaglio N, Gugliotta A, Tardivo MB, et al. Improvement of in vitro stability and pharmacokinetics of hIFN-α by fusing the carboxyl-terminal peptide of hCG β-subunit. J Biotechnol, 2016, 221: 13–24
Mohammed Y, EL-Baky NA, Redwan EM. Expression, purification, and characterization of recombinant human consensus interferon-alpha in Escherichia coli under λPL promoter. Preparative Biochem Biotechnol, 2012, 42: 426–447
Park IH, Baek KW, Cho EY, et al. PKR-dependent mechanisms of interferon-α for inhibiting hepatitis B virus replication. Mol Cells, 2011, 32: 167–172
Koci’c G, Koci’c R, Vlahovi’c P, et al. Different responses of rat liver adenosine metabolizing enzymes during in vivo and in vitro treatment with interferon-alpha2b. J Viral Hepat, 1998, 5: 353–356
Sakatoku K, Nakashima Y, Nagasaki J, et al. Immunomodulatory and direct activities of ropeginterferon alfa-2b on cancer cells in mouse models of leukemia. Cancer Sci, 2022, 113: 2246–2257
Bukowski RM. Pegylated interferon alfa-2b as treatment of solid tumors. Curr Oncol Rep, 2003, 5: 87–88
McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med, 2009, 361: 580–593
Bex A, Mallo H, Kerst M, et al. A phase-II study of pegylated interferon alfa-2b for patients with metastatic renal cell carcinoma and removal of the primary tumor. Cancer Immunol Immunother, 2005, 54: 713–719
Leader B, Baca QJ, Golan DE. Protein therapeutics: A summary and pharmacological classification. Nat Rev Drug Discov, 2008, 7: 21–39
El-Baky NA, Redwan EM. Therapeutic alpha-interferons protein: Structure, production, and biosimilar. Preparative Biochem Biotechnol, 2015, 45: 109–127
Zeinali S, Shahrokhi M, Ibeas A. Observer-based impulsive controller design for treatment of hepatitis C disease. Ind Eng Chem Res, 2020, 59: 19370–19382
Borden EC, Sen GC, Uze G, et al. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov, 2007, 6: 975–990
Obenauer-Kutner LJ, Jacobs SJ, Kolz K, et al. A highly sensitive electrochemiluminescence immunoassay for interferon alfa-2b in human serum. J Immunol Methods, 1997, 206: 25–33
Vrolijk JM, Kaul A, Hansen BE, et al. A replicon-based bioassay for the measurement of interferons in patients with chronic hepatitis C. J Virological Methods, 2003, 110: 201–209
Na DH, Park EJ, Youn YS, et al. Sodium dodecyl sulfate-capillary gel electrophoresis of polyethylene glycolylated interferon alpha. Electro-phoresis, 2004, 25: 476–479
Diress A, Lorbetskie B, Larocque L, et al. Study of aggregation, dena-turation and reduction of interferon alpha-2 products by size-exclusion high-performance liquid chromatography with fluorescence detection and biological assays. J Chromatography A, 2010, 1217: 3297–3306
Saylan Y, Akgönüllü S, Denizli A. Preparation of magnetic nano-particles-assisted plasmonic biosensors with metal affinity for inter-feron-α detection. Mater Sci Eng-B, 2022, 280: 115687
Bie Z, Chen Y. Selective analysis of interferon-alpha in human serum with boronate affinity oriented imprinting based plastic antibody. Talanta, 2021, 230: 122338
Santana H, Espino Y, Franco A, et al. A sandwich-type enzyme-linked immunosorbent assay for the analysis of recombinant human interferon alpha-2b. Biotechnol Tech, 1999, 13: 341–346
Mundim FV, Trovo MA, Stark LM, et al. Pegylated-interferon-alpha treatment modifying T cell cytokine profile in tumor microenvironment of patients with cervical intraepithelial neoplasia. Eur J Gynaecol Oncol, 2021, 42: 96–104
Shaaban R, El-Sayed WM, Samir S, et al. Molecular and biological characterization of a prepared recombinant human interferon alpha 2b isoform. Appl Biochem Biotechnol, 2019, 188: 72–86
Bhattacharya D, Baksi A, Banerjee I, et al. Development of phosphonate modified Fe(1−x)MnxFe2O4 mixed ferrite nanoparticles: Novel peroxidase mimetics in enzyme linked immunosorbent assay. Talanta, 2011, 86: 337–348
Palzer J, Eckstein L, Slabu I, et al. Iron oxide nanoparticle-based hyperthermia as a treatment option in various gastrointestinal malignancies. Nanomaterials, 2021, 11: 3013
Kim BH, Lee N, Kim H, et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J Am Chem Soc, 2011, 133: 12624–12631
Zhang T, Xu Q, Huang T, et al. New insights into biocompatible iron oxide nanoparticles: A potential booster of gene delivery to stem cells. Small, 2020, 16: 2001588
Wu Y, Lu Z, Li Y, et al. Surface modification of iron oxide-based magnetic nanoparticles for cerebral theranostics: Application and prospection. Nanomaterials, 2020, 10: 1441
Muley AB, Mulchandani KH, Singhal RS. Immobilization of enzymes on iron oxide magnetic nanoparticles: Synthesis, characterization, kinetics and thermodynamics. Methods Enzymol, 2020, 630: 39–79
Tang D, Su B, Tang J, et al. Nanoparticle-based sandwich electrochemical immunoassay for carbohydrate antigen 125 with signal enhancement using enzyme-coated nanometer-sized enzyme-doped silica beads. Anal Chem, 2010, 82: 1527–1534
Gao L, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotech, 2007, 2: 577–583
Yang M, Guan Y, Yang Y, et al. Peroxidase-like activity of amino-functionalized magnetic nanoparticles and their applications in immunoassay. J Colloid Interface Sci, 2013, 405: 291–295
Gilbride B, Schmidt Garcia Moreira GM, Hust M, et al. Catalytic ferromagnetic gold nanoparticle immunoassay for the detection and differentiation of Mycobacterium tuberculosis and Mycobacterium bovis. Anal Chim Acta, 2021, 1184: 339037
Li H, Kou B, Yuan Y, et al. Porous Fe3O4@COF-immobilized gold nanoparticles with excellent catalytic performance for sensitive electrochemical detection of ATP. Biosens Bioelectron, 2022, 197: 113758
Chen Q, Rong S, Cen Y, et al. A facile fluorescent sensor based on carbon dots and Fe3O4 nanoplates for the detection of hyaluronidase activity. Sens Actuat B-Chem, 2021, 346: 130434
Li M, Li J, Zhang X, et al. Simultaneous detection of tumor-related mRNA and miRNA in cancer cells with magnetic SERS nanotags. Talanta, 2021, 232: 122432
Lee JH, Choi HK, Lee SY, et al. Enhancing immunoassay detection of antigens with multimeric protein Gs. Biosens Bioelectron, 2011, 28: 146–151
Kim Y, Choi YS, Lee HJ, et al. Self-assembly of fluorescent and magnetic Fe3O4@coordination polymer nanochains. Chem Commun, 2014, 50: 7617–7620
Chen J, Gao H, Li Z, et al. Ferriporphyrin-inspired MOFs as an artificial metalloenzyme for highly sensitive detection of H2O2 and glucose. Chin Chem Lett, 2020, 31: 1398–1401
Luo Z, Qi Q, Zhang L, et al. Branched polyethylenimine-modified upconversion nanohybrid-mediated photoelectrochemical immunoassay with synergistic effect of dual-purpose copper ions. Anal Chem, 2019, 91: 4149–4156
Liu H, Li Z, Shen R, et al. Point-of-care pathogen testing using photonic crystals and machine vision for diagnosis of urinary tract infections. Nano Lett, 2021, 21: 2854–2860
Guo H, Su X, Su Q, et al. Au-coated Fe3O4 core-shell nanohybrids with photothermal activity for point-of-care immunoassay for lipoprotein-associated phospholipase A2 on a digital near-infrared thermometer. Anal Bioanal Chem, 2021, 413: 235–244
Qin Q, Li H, Shi X, et al. Facile synthesis of Fe3O4@polyethyleneimine modified with 4-formylphenylboronic acid for the highly selective extraction of major catecholamines from human urine. J Sep Sci, 2015, 38: 2857–2864
Zhu H, Liu C, Liu X, et al. A multi-colorimetric immunosensor for visual detection of ochratoxin A by mimetic enzyme etching of gold nanobipyramids. Microchim Acta, 2021, 188: 62
Yu C, Li Q, Tian J, et al. A facile preparation of immobilized nar-inginase on polyethyleneimine-modified Fe3O4 magnetic nanomaterials with high activity. RSC Adv, 2021, 11: 14568–14577
You L, Huang C, Lu F, et al. Facile synthesis of high performance porous magnetic chitosan-polyethylenimine polymer composite for Congo red removal. Int J Biol Macromolecules, 2018, 107: 1620–1628
Zhang S, Wang Z, Chen H, et al. Polyethylenimine functionalized Fe3O4/steam-exploded rice straw composite as an efficient adsorbent for Cr(VI) removal. Appl Surf Sci, 2018, 440: 1277–1285
Mdlovu NV, Chen Y, Lin KS, et al. Multifunctional nanocarrier as a potential micro-RNA delivery vehicle for neuroblastoma treatment. J Taiwan Institute Chem Engineers, 2019, 96: 526–537
Li J, Wang C, Shi L, et al. Rapid identification and antibiotic susceptibility test of pathogens in blood based on magnetic separation and surface-enhanced Raman scattering. Microchim Acta, 2019, 186: 475
Hu J, Liu L, Danielsson B, et al. Piezoelectric immunosensor for detection of complement C6. Anal Chim Acta, 2000, 423: 215–219
Tao N, Xu Y, Wang L, et al. Hollow porous N-doped carbon-based Co4N with peroxidase-like activity for detection of H2O2 under non-physiologic conditions. MicroChem J, 2021, 166: 106206
Qiang W, Li W, Li X, et al. Bioinspired polydopamine nanospheres: A superquencher for fluorescence sensing of biomolecules. Chem Sci, 2014, 5: 3018–3024
Zhang K, Wang K, Huang Y, et al. Sensitive detection of cytokine in complex biological samples by using MB track mediated DNA walker and nicking enzyme assisted signal amplification method combined biosensor. Talanta, 2018, 189: 122–128
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFA0709202), the Natural Science Foundation of Jilin Province (20220101055JC), the International Cooperation Project of Jilin Scientific and Technological Development Program (20190701059GH), and the Department of Science and Technology of Jilin Province (20220508098RC).
Author information
Authors and Affiliations
Contributions
Author contributions Sun X conducted the experiments and drafted the original manuscript; Chang J and Zhang X provided direct guidance in ELISA investigation and contributed to manuscript revision; Dong Q, Wang H and Gao H were involved in the characterization of nanomaterials and data analysis; Wang E and Li D were responsible for securing the funding for the project; Li D and Wang J contributed to the design, supervision, and manuscript revision.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Xu Sun received her Master’s degree from Jilin University and studied as an exchange student at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (CAS). Her current research focuses on the synthesis of functional nanomaterials, including nanozymes, as well as the design and development of biosensors.
Dan Li is an associate professor at Changchun Institute of Applied Chemistry, CAS. She received her PhD degree from Jilin University, China, in 2015. In 2017, she worked as a visiting researcher at Macquarie University, Australia. Her primary research interests lie in the development of innovative nanomaterials for applications in analytical chemistry and cancer theranostics.
Haicheng Gao is a professor at Jilin University, where he has been conducting research since earning his PhD degree from the same institution in 2009. He also served as an associate professor at the National University of Singapore in 2017. His research primarily centers on molecular clinical pharmacy. Additionally, he is actively involved in the synthesis of nanomaterials and nanome-dicines.
Jin Wang earned his PhD degree from the University of Illinois in 1991. Following that, he held positions as a postdoctoral research fellow and visiting researcher in chemistry and biophysics at the University of Illinois and the National Institutes of Health (NIH). Currently, he serves as a professor of the Department of Chemistry and the Department of Physics and Astronomy at the State University of New York at Stony Brook. His research focuses on biophysics, statistical physics, analytical chemistry, and materials science.
Supplementary information Supporting data are available in the online version of the paper.
Supplementary Information
40843_2023_2549_MOESM1_ESM.pdf
Facile synthesis of Fe3O4@polyethylenimine with peroxidase-like activity for highly sensitive detection of interferon α-2b
Rights and permissions
About this article
Cite this article
Sun, X., Chang, J., Dong, Q. et al. Facile synthesis of Fe3O4@polyethylenimine with peroxidase-like activity for highly sensitive detection of interferon α-2b. Sci. China Mater. 66, 4121–4130 (2023). https://doi.org/10.1007/s40843-023-2549-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2549-3