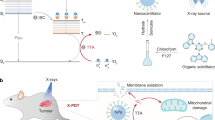
Abstract
X-ray nanoscintillator-based photoresponsive therapy is emerging as a promising strategy to treat in vivo tumors, necessitating the development of nanoscintillators with X-ray-excited luminescence in the ultraviolet region (UV-XEL) for the photoactivation of therapeutic precursors. However, it remains a challenge to achieve high-efficiency X-ray-activated tumor therapy due to the limited UV-XEL efficacy. Herein, we developed novel Gd3+/Ce3+-codoped LiLuF4 nanoscintillators with strong UV-XEL for in vivo tumor therapy. Significantly, through optimization of host materials, dopants, and energy transfer process, the developed nanoscintillators enabled effective enhancement of UV-XEL (up to 18-fold) as compared with the traditional Ce3+-doped counterparts. As a proof-of-concept study to evaluate the in vivo therapeutic applications of the nanoscintillators, we integrated them with nitric oxide (NO) precursors for controllably generating NO upon the X-ray exposure, achieving superior in vivo antitumor effect upon X-ray-induced synergetic NO therapy and radiotherapy. Moreover, X-ray-activated NO therapy can inhibit tumor metastasis into liver to suppress tumor regrowth and prolong mice survival. This work might motivate future development of X-ray nanoscintillators for treating diseases in deep tissue.

摘要
基于X射线纳米闪烁体的光响应疗法是一种新兴的有良好应用前景的活体肿瘤治疗策略, 迫切需要开发具有高效X射线激发紫外发光性能的纳米闪烁体. 然而, 由于当前纳米闪烁体的X射线紫外发光性能仍较弱, 实现高效的X射线激活肿瘤治疗仍然是一个巨大的挑战. 为此, 我们发展了一种新型的具有良好X射线紫外发光性能的Gd3+/Ce3+共掺杂的LiLuF 4 纳米闪烁体, 并将其应用于活体肿瘤治疗. 通过优化基质材料、 掺杂剂和能量传递设计合成的纳米闪烁体, 其X射线紫外发光强度比传统的Ce3+单掺杂材料增强了约18倍. 我们进一步将纳米闪烁体与一氧化氮(NO)前体结合以概念验证研究纳米闪烁体的应用. 在X射线照射下, 该纳米复合物能够可控地产生NO, 并在X射线诱导的NO和放射治疗协同作用下实现了优异的抗肿瘤效果. 此外, X射线激活的NO治疗可以抑制肿瘤向肝脏转移, 抑制肿瘤再生, 延长小鼠存活率. 这项工作有望推动纳米闪烁体的发展及其在活体深层组织疾病治疗中的应用.
Similar content being viewed by others
References
McGregor DS. Materials for gamma-ray spectrometers: Inorganic scintillators. Annu Rev Mater Res, 2018, 48: 245–277
Ashworth C. Super scintillators. Nat Rev Mater, 2018, 3: 355
Zhou Y, Chen J, Bakr OM, et al. Metal halide perovskites for X-ray imaging scintillators and detectors. ACS Energy Lett, 2021, 6: 739–768
Li Z, Zhou F, Yao HH, et al. Halide perovskites for high-performance X-ray detector. Mater Today, 2021, 48: 155–175
Chen Q, Wu J, Ou X, et al. All-inorganic perovskite nanocrystal scintillators. Nature, 2018, 561: 88–93
Zhou Z, Song J, Nie L, et al. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev, 2016, 45: 6597–6626
Liang H, Hong Z, Li S, et al. An activatable X-ray scintillating luminescent nanoprobe for early diagnosis and progression monitoring of thrombosis in live rat. Adv Funct Mater, 2021, 31: 2006353
Sun W, Zhou Z, Pratx G, et al. Nanoscintillator-mediated X-ray induced photodynamic therapy for deep-seated tumors: From concept to biomedical applications. Theranostics, 2020, 10: 1296–1318
He L, Yu X, Li W. Recent progress and trends in X-ray-induced photodynamic therapy with low radiation doses. ACS Nano, 2022, 16: 19691–19721
Lu L, Sun M, Lu Q, et al. High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy. Nano Energy, 2021, 79: 105437
Fan W, Tang W, Lau J, et al. Breaking the depth dependence by nanotechnology-enhanced X-ray-excited deep cancer theranostics. Adv Mater, 2019, 31: 1806381
Fan W, Yung BC, Chen X. Stimuli-responsive NO release for on-demand gas-sensitized synergistic cancer therapy. Angew Chem Int Ed, 2018, 57: 8383–8394
Xie Z, Fan T, An J, et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev, 2020, 49: 8065–8087
Zhou S, Li D, Lee C, et al. Nanoparticle phototherapy in the era of cancer immunotherapy. Trends Chem, 2020, 2: 1082–1095
Chen Q, Chen M, Liu Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem Soc Rev, 2019, 48: 5506–5526
Zheng L, Zhu R, Chen L, et al. X-ray sensitive high-Z metal nanocrystals for cancer imaging and therapy. Nano Res, 2021, 14: 3744–3755
Zhang Y, Xu C, Yang X, et al. Photoactivatable protherapeutic nanomedicine for cancer. Adv Mater, 2020, 32: 2002661
Du Z, Zhang X, Guo Z, et al. X-ray-controlled generation of peroxynitrite based on nanosized LiLuF4:Ce3+ scintillators and their applications for radiosensitization. Adv Mater, 2018, 30: 1804046
Grajcarek J, Monlong J, Nishinaka-Arai Y, et al. Genome-wide microhomologies enable precise template-free editing of biologically relevant deletion mutations. Nat Commun, 2019, 10: 4856
Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics, 2013, 3: 317–330
Li J, Duan H, Pu K. Nanotransducers for near-infrared photoregulation in biomedicine. Adv Mater, 2019, 31: 1901607
Chen X, Song J, Chen X, et al. X-ray-activated nanosystems for theranostic applications. Chem Soc Rev, 2019, 48: 3073–3101
Ding B, Sheng J, Zheng P, et al. Biodegradable upconversion nanoparticles induce pyroptosis for cancer immunotherapy. Nano Lett, 2021, 21: 8281–8289
Bünzli JCG. Lanthanide-doped nanoscintillators. Light Sci Appl, 2022, 11: 285
Jiang M, Deng Z, Zeng S, et al. Recent progress on lanthanide scintillators for soft X-ray-triggered bioimaging and deep-tissue theranostics. VIEW, 2021, 2: 20200122
Ou X, Qin X, Huang B, et al. High-resolution X-ray luminescence extension imaging. Nature, 2021, 590: 410–415
Matsubara T, Yanagida T, Kawaguchi N, et al. Remote control of neural function by X-ray-induced scintillation. Nat Commun, 2021, 12: 4487
Ning Y, Zhu M, Zhang JL. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord Chem Rev, 2019, 399: 213028
Squillante MR, Jüstel T, Anderson RR, et al. Fabrication and characterization of UV-emitting nanoparticles as novel radiation sensitizers targeting hypoxic tumor cells. Optical Mater, 2018, 80: 197–202
Nakamura F, Kato T, Okada G, et al. Scintillation, dosimeter and optical properties of MgF2 transparent ceramics doped with Gd3+. Mater Res Bull, 2018, 98: 83–88
Bulin A, Broekgaarden M, Chaput F, et al. Radiation dose-enhancement is a potent radiotherapeutic effect of rare-earth composite nanoscintillators in preclinical models of glioblastoma. Adv Sci, 2020, 7: 2001675
Bartley AF, Fischer M, Bagley ME, et al. Feasibility of cerium-doped LSO particles as a scintillator for X-ray induced optogenetics. J Neural Eng, 2021, 18: 046036
Wang H, Lv B, Tang Z, et al. Scintillator-based nanohybrids with sacrificial electron prodrug for enhanced X-ray-induced photodynamic therapy. Nano Lett, 2018, 18: 5768–5774
Liu S, Fang L, Ding H, et al. Alternative strategy to optimize cerium oxide for enhanced X-ray-induced photodynamic therapy. ACS Nano, 2022, 16: 20805–20819
Liu S, Li W, Zhang Y, et al. Tailoring silica-based nanoscintillators for peroxynitrite-potentiated nitrosative stress in postoperative radiotherapy of colon cancer. Nano Lett, 2022, 22: 6409–6417
Du Z, Wang X, Zhang X, et al. X-ray-triggered carbon monoxide and manganese dioxide generation based on scintillating nanoparticles for cascade cancer radiosensitization. Angew Chem Int Ed, 2023, 62: e202302525
Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med, 2006, 12: 895–904
Bakir B, Chiarella AM, Pitarresi JR, et al. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol, 2020, 30: 764–776
Zhang Z, Wang H, Tan T, et al. Rational design of nanoparticles with deep tumor penetration for effective treatment of tumor metastasis. Adv Funct Mater, 2018, 28: 1801840
Kong X, Cheng R, Wang J, et al. Nanomedicines inhibiting tumor metastasis and recurrence and their clinical applications. Nano Today, 2021, 36: 101004
Pokorný M, Babin V, Beitlerová A, et al. Gd-admixed (Lu,Gd)AlO3 single crystals: Breakthrough in heavy perovskite scintillators. NPG Asia Mater, 2021, 13: 66
Sun B, Xie Y, Zhao Y, et al. A highly robust Ce3+-doped and Gd3+-mixed KLaF4 nano-glass composite scintillator. J Mater Chem C, 2021, 9: 17504–17510
Lei L, Wang Y, Xu W, et al. Manipulation of time-dependent multicolour evolution of X-ray excited afterglow in lanthanide-doped fluoride nanoparticles. Nat Commun, 2022, 13: 5739
Thoma RE, Insley H, Hebert GM. The sodium fluoride-lanthanide trifluoride systems. Inorg Chem, 1966, 5: 1222–1229
Richard C, Viana B. Persistent X-ray-activated phosphors: Mechanisms and applications. Light Sci Appl, 2022, 11: 123
Naccache R, Yu Q, Capobianco JA. The fluoride host: Nucleation, growth, and upconversion of lanthanide-doped nanoparticles. Adv Opt Mater, 2015, 3: 482–509
Huang P, Zheng W, Tu D, et al. Unraveling the electronic structures of neodymium in LiLuF4 nanocrystals for ratiometric temperature sensing. Adv Sci, 2019, 6: 1802282
Maurizio SL, Mandl GA, Long MD, et al. Investigating the fundamental material properties that influence the radioluminescence of lanthanide-doped nanoparticles. Chem Mater, 2022, 34: 10123–10132
Shalapska T, Stryganyuk G, Demchenko P, et al. Luminescence properties of Ce3+-doped LiGdP4O12 upon vacuum-ultraviolet and X-ray excitation. J Phys-Condens Matter, 2009, 21: 445901
Demchenko P, Gektin A, Krasnikov A, et al. Energy migration and Gd3+ ↔ Ce3+ transfer in Ce3+-doped GdP3O9 metaphosphate. J Phys D-Appl Phys, 2013, 46: 235103
Kucera M, Rathaiah M, Beitlerova A, et al. Scintillation properties and energy transfer in (GdY)AlO3Ce3+ perovskites with high Gd content. IEEE Trans Nucl Sci, 2020, 67: 1049–1054
Bartosiewicz K, Babin V, Kamada K, et al. Energy migration processes in undoped and Ce-doped multicomponent garnet single crystal scintillators. J Lumin, 2015, 166: 117–122
Rathaiah M, Kucera M, Pejchal J, et al. Epitaxial growth, photoluminescence and scintillation properties of Gd3+ co-doped YAlO3:Ce3+ films. Radiat Measurements, 2019, 121: 86–90
Xu Y, Liu J, Liu Z, et al. Blockade of platelets using tumor-specific NO-releasing nanoparticles prevents tumor metastasis and reverses tumor immunosuppression. ACS Nano, 2020, 14: 9780–9795
Li S, Li L, Lin X, et al. Targeted inhibition of tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates cancer metastasis. ACS Nano, 2021, 16: 50–67
Shi M, Zhang J, Wang Y, et al. Tumor-specific nitric oxide generator to amplify peroxynitrite based on highly penetrable nanoparticles for metastasis inhibition and enhanced cancer therapy. Biomaterials, 2022, 283: 121448
Salvati A, Åberg C, dos Santos T, et al. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics. Nanomed-Nanotechnol Biol Med, 2011, 7: 818–826
Song X, Li S, Guo H, et al. Graphene-oxide-modified lanthanide nanoprobes for tumor-targeted visible/NIR-II luminescence imaging. Angew Chem Intl Edit, 2019, 58: 18981–18986
Song L, Li PP, Yang W, et al. Low-dose X-ray activation of W(VI)-doped persistent luminescence nanoparticles for deep-tissue photodynamic therapy. Adv Funct Mater, 2018, 28: 1707496
Cheng S, Liu L, Yang Q, et al. In vivo optical bioimaging by using Nd-doped LaF3 luminescent nanorods in the second near-infrared window. J Rare Earths, 2019, 37: 931–936
Li S, Wei J, Yao Q, et al. Emerging ultrasmall luminescent nanoprobes for in vivo bioimaging. Chem Soc Rev, 2023, 52: 1672–1696
Fan C, Wang Q, van der Zon G, et al. OVOL1 inhibits breast cancer cell invasion by enhancing the degradation of TGF-β type I receptor. Sig Transduct Target Ther, 2022, 7: 126
Yu L, Hu P, Chen Y. Gas-generating nanoplatforms: Material chemistry, multifunctionality, and gas therapy. Adv Mater, 2018, 30: 1801964
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22027805 and 22274024), the Major Project of Science and Technology of Fujian Province (2020HZ06006), the Young Elite Scientist Sponsorship Program by CAST (YESS20200110), and China Postdoctoral Science Foundation (2022M720737 and 2021T140117)
Author information
Authors and Affiliations
Contributions
Author contributions Song X conceived the idea and supervised this work. Yang K, Yang Y, and Sun D conducted the experiments, analyzed the data, and prepared the manuscript. Li S, Song X, and Yang H revised the manuscript. All authors contributed to the general discussion.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Kaidong Yang is a Master candidate at Fuzhou University. His current research focuses on the lanthanide-doped nanoscintillators.
Xiaorong Song is an associate professor at the college of chemistry, Fuzhou University. He received his PhD degree in 2017 from Fuzhou University, and continued his visiting scholar study at the National University of Singapore (NUS) and postdoctoral research at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences (China). His research interests focus on the luminescent nanomaterials and bioapplications.
Supplementary information Supporting data are available in the online version of the paper.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, K., Yang, Y., Sun, D. et al. Designing highly UV-emitting lanthanide nanoscintillators for in vivo X-ray-activated tumor therapy. Sci. China Mater. 66, 4090–4099 (2023). https://doi.org/10.1007/s40843-023-2548-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2548-8