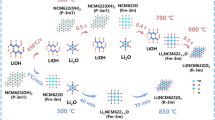
Abstract
Lithium-rich layered oxides (LROs) are regarded as promising cathode materials to build high-energy-density lithium-ion batteries (LIBs). However, conventional polycrystalline LROs suffer from irreversible structure changes and slow interfacial kinetics, leading to poor cycle and rate performance. Here we propose a polyvinylpyrrolidone (PVP)-assisted co-precipitation method to prepare single-crystal LRO (Li1.2Mn0.54Ni0.13Co0.13O2) nanosheets. PVP can adsorb on a specific crystal plane during precursor formation to obtain ideal nanosheet morphology. This method is simple, low-cost and easy to scale up. The prepared single-crystal nanosheets feature continuous lattice and no grain boundary inside, which shorten the path of Li+ intercalation/deintercalation and improve the electrode reaction kinetics. The single-crystal structure also inhibits the irreversible phase transformation from the layered phase to the spinel phase and the formation of cracks owing to suitable particle size, stabilizing the layered structure. As a result, the prepared single-crystal Li1.2Mn0.54-Ni0.13Co0.13O2 nanosheets deliver a reversible capacity of 254.5 mA h g−1 at a rate of 0.1 C and good cycling stability with a capacity retention of 71.9% after 1000 cycles at a high rate of 5 C. This work provides a facile method to prepare nano-sized single-crystal LRO materials for improving the cycle and rate performance of LIBs.

摘要
富锂层状氧化物是构筑高能量密度锂离子电池富有潜力的正极材料. 然而, 由于不可逆的结构变化和缓慢的界面动力学, 传统的多晶富锂层状氧化物正极材料循环和倍率性能较差. 本文提出了一种聚乙烯基吡咯烷酮(PVP-K30)辅助共沉淀制备单晶Li1.2Mn0.54Ni0.13Co0.13O2纳米片的方法. 这种方法操作简单、成本低且便于放大生产. 所制备的单晶纳米片内部晶格连续且无晶界, 缩短了Li+的嵌入/脱嵌路径, 加快了电极反应动力学过程. 单晶结构还能抑制层状相向尖晶石相的不可逆相变和颗粒内部裂纹的形成, 起到稳定层状结构的作用. 电化学测试结果表明, 所制备的Li1.2Mn0.54Ni0.13Co0.13O2单晶纳米片在0.1 C倍率下的可逆容量为254.5 mA h g−1, 在5 C高倍率下循环1000次后容量保持率为71.9%. 这种简单的制备纳米片单晶材料的方法为提高富锂层状氧化物正极材料的循环性能和倍率性能提供了新的思路.
Similar content being viewed by others
References
Zhang K, Hu Z, Tao Z, et al. Inorganic & organic materials for rechargeable Li batteries with multi-electron reaction. Sci China Mater, 2014, 57: 42–58
Hua W, Yang X, Casati NPM, et al. Probing thermally-induced structural evolution during the synthesis of layered Li-, Na-, or K-containing 3d transition-metal oxides. eScience, 2022, 2: 183–191
Feng J, Jiang YS, Yu FD, et al. Understanding Li roles in chemical reversibility of O2-type Li-rich layered cathode materials. J Energy Chem, 2022, 66: 666–675
Xu YS, Zhou YN, Zhang QH, et al. Layered oxides with solid-solution reaction for high voltage potassium-ion batteries cathode. Chem Eng J, 2021, 412: 128735
Li W, Zeng L, Wu Y, et al. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning. Sci China Mater, 2016, 59: 287–321
Liu S, Yu Z, Wang Y, et al. Catalytic dehydration of glycerol to acrolein over unsupported MoP. Catal Today, 2021, 379: 132–140
Xie J, Lu YC. A retrospective on lithium-ion batteries. Nat Commun, 2020, 11: 2499
Gao A, Li X, Zhang Q, et al. Critical intermediate β-Li2NiO3 phase for structural degradation of Ni-rich layered cathodes during thermal runaway. Battery Energy, 2023, 2: 20220036
Hua W, Wang S, Knapp M, et al. Structural insights into the formation and voltage degradation of lithium- and manganese-rich layered oxides. Nat Commun, 2019, 10: 5365
Gou X, Hao Z, Hao Z, et al. In situ surface self-reconstruction strategies in Li-rich Mn-based layered cathodes for energy-dense Li-ion batteries. Adv Funct Mater, 2022, 32: 2112088
Cui H, Li H, Liu J, et al. Surface modification of Li-rich manganese-based cathode materials by chemical etching. Inorg Chem Front, 2019, 6: 1694–1700
Zhang J, Cheng F, Chou S, et al. Tuning oxygen redox chemistry in Li-rich Mn-based layered oxide cathodes by modulating cation arrangement. Adv Mater, 2019, 31: 1901808
Wang J, He X, Paillard E, et al. Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries. Adv Energy Mater, 2016, 6: 1600906
Nie L, Wang Z, Zhao X, et al. Cation/anion codoped and cobalt-free Li-rich layered cathode for high-performance Li-ion batteries. Nano Lett, 2021, 21: 8370–8377
Wang Y, Wang E, Zhang X, et al. High-voltage “single-crystal” cathode materials for lithium-ion batteries. Energy Fuels, 2021, 35: 1918–1932
Li Q, Ning D, Wong D, et al. Improving the oxygen redox reversibility of Li-rich battery cathode materials via Coulombic repulsive interactions strategy. Nat Commun, 2022, 13: 1123
You B, Wang Z, Shen F, et al. Research progress of single-crystal nickel-rich cathode materials for lithium ion batteries. Small Methods, 2021, 5: 2100234
He W, Guo W, Wu H, et al. Challenges and recent advances in high capacity Li-rich cathode materials for high energy density lithium-ion batteries. Adv Mater, 2021, 33: 2005937
Yin S, Deng W, Chen J, et al. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy, 2021, 83: 105854
Wang E, Xiao D, Wu T, et al. Stabilizing oxygen by high-valance element doping for high-performance Li-rich layered oxides. Battery Energy, 2023, 2: 20220030
Sun J, Sheng C, Cao X, et al. Restraining oxygen release and suppressing structure distortion in single-crystal Li-rich layered cathode materials. Adv Funct Mater, 2022, 32: 2110295
Zheng L, Bennett JC, Obrovac MN. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries. J Electrochem Soc, 2020, 167: 130536
Zhu J, Chen G. Single-crystal based studies for correlating the properties and high-voltage performance of Li[NixMnyCo1−x−y]O2 cathodes. J Mater Chem A, 2019, 7: 5463–5474
Gao Y, Wang Z, Liu SX, et al. Influence of anions on the morphology of nanophase α-MnO2 crystal via hydrothermal process. J Nanosci Nanotech, 2006, 6: 2576–2579
Li J, Li H, Stone W, et al. Synthesis of single crystal LiNi0.5Mn0.3Co0.2O2 for lithium ion batteries J Electrochem Soc, 2017, 164: A3529–A3537
Luo D, Li G, Fu C, et al. LiMO2 (M = Mn, Co, Ni) hexagonal sheets with (101) facets for ultrafast charging-discharging lithium ion batteries J Power Sources, 2015, 276: 238–246
Min Y, Moon GD, Kim BS, et al. Quick, controlled synthesis of ultrathin Bi2Se3 nanodiscs and nanosheets. J Am Chem Soc, 2012, 134: 2872–2875
Wang Y, Wang L, Guo X, et al. Thermal stability enhancement through structure modification on the microsized crystalline grain surface of lithium-rich layered oxides ACS Appl Mater Interfaces, 2020, 12: 8306–8315
Mizuno Y, Zettsu N, Yubuta K, et al. Fabrication of LiCoO2 crystal layers using a flux method and their application for additive-free lithium-ion rechargeable battery cathodes Cryst Growth Des, 2014, 14: 1882–1887
Intarabumrung W, Kuntharin S, Harnchana V, et al. Facile synthesis of biobased polyamide derived from epoxidized soybean oil as a high-efficiency triboelectric nanogenerator ACS Sustain Chem Eng, 2022, 10: 13680–13691
Pitsevich GA, Malevich AE, Kozlovskaya EN, et al. Theoretical study of the C-H/O-H stretching vibrations in malonaldehyde SpectroChim Acta Part A-Mol Biomol Spectr, 2015, 145: 384–393
Khare BN, Meyyappan M, Cassell AM, et al. Functionalization of carbon nanotubes using atomic hydrogen from a glow discharge Nano Lett, 2002, 2: 73–77
Pişkin B, Savaş Uygur C, Aydınol MK. Mo doping of layered Li(NixMnyCo1−x−y−zMz)O2 cathode materials for lithium-ion batteries. Int J Energy Res, 2018, 42: 3888–3898
Zhao J, Huang R, Gao W, et al. An ion-exchange promoted phase transition in a Li-excess layered cathode material for high-performance lithium ion batteries. Adv Energy Mater, 2015, 5: 1401937
Ye Z, Zhang B, Chen T, et al. A simple gas-solid treatment for surface modification of Li-rich oxides cathodes. Angew Chem Int Ed, 2021, 60: 23248–23255
Yi L, Liu Z, Yu R, et al. Li-rich layered/spinel heterostructured special morphology cathode material with high rate capability for Li-ion batteries. ACS Sustain Chem Eng, 2017, 5: 11005–11015
Cao W, Yan J, Zhang P, et al. Cerium-doped lithium-rich Li1.2Mn0.56-Ni0.11Co0.13O2 as cathode with high performance for lithium-ion batteries Ionics, 2022, 28: 4515–4526
Cui SL, Xiao ZX, Cui BC, et al. Fast charge-transport interface on primary particles boosts high-rate performance of Li-rich Mn-based cathode materials ACS Appl Mater Interfaces, 2023, 15: 13195–13204
Bi S, Wang S, Yue F, et al. A rechargeable aqueous manganese-ion battery based on intercalation chemistry Nat Commun, 2021, 12: 6991
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22121005, 22020102002 and 21835004) and the Frontiers Science Center for New Organic Matter of Nankai University (63181206). We also appreciate Tianjin Lishen New Energy Technology Co., Ltd. for financial support
Author information
Authors and Affiliations
Contributions
Chen J and Yan Z proposed the idea of this work. Hao Z and Gou X conducted the experiments. Hao Z and Gou X wrote the manuscript with support from Chen J, Yan Z, Zhao Q, Ma H, Lu Y, Jin H, and Zhang Q. Hao Z, Yang Z and Yang G organized the figures. The manuscript was discussed and revised by all authors.
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Supplementary information
Supporting data are available in the online version of the paper.
Zhenkun Hao received his BS degree in applied chemistry from Qingdao University of Science and Technology (2020). He is currently a master candidate at the Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University under the supervision of Prof. Jun Chen. His research activities focus on high-voltage cathode materials in LIBs.
Zhenhua Yan is an associate professor at the College of Chemistry, Nankai University. He obtained his PhD from Nankai University in 2018 under the supervision of Prof. Fangyi Cheng. Then, he joined the College of Chemistry, Nankai University as a master’s supervisor and assistant of Prof. Jun Chen. His research interests involve the design and synthesis of nanomaterials for batteries and electrocatalysis.
Jun Chen obtained his BS and MS degrees from Nankai University (Tianjin) in 1989 and 1992, respectively. He received his PhD degree from the University of Wollongong (Australia) in 1999. His research mainly focuses on the synthetic chemistry of inorganic and organic solid functional materials and the development of batteries.
Rights and permissions
About this article
Cite this article
Hao, Z., Gou, X., Ma, H. et al. Boosting the cycle and rate performance of Li1.2Mn0.54Ni0.13Co0.13O2 via single-crystal structure design. Sci. China Mater. 66, 3424–3432 (2023). https://doi.org/10.1007/s40843-023-2494-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2494-1