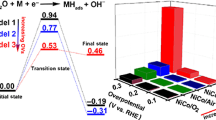
Abstract
Electron configurations and conductivity are significant descriptors/characteristics for the oxygen evolution reaction (OER), which can be modulated with heteroatom doping. Given that metal substitution usually reduces the number of active sites of spinel electrocatalysts, the effect of anion doping on the electronic structure has been investigated by using ZnCo2O4 (ZCO) as a demonstration. Compared with Co3+-dominated ZCO, the substitution of oxygen with less electronegative sulfur raises the portion of Co2+ in the low-spin states (t2g6eg1), which is more OER-active than Co3+ (t2g6eg0). Co2+ in the sulfur-doped ZCO (ZCO-S) is associated with the redistribution of electron density from S toward Co due to the high covalent interaction of Co-S. The Co-S interaction also induces a fast charge transfer. ZCO-S outperforms pristine ZCO by 11 times in terms of specific OER activity at 1.65 V versus reversible hydrogen electrode. In contrast, doping fluorine with higher electronegativity and valence deteriorates OER activity. Our work establishes the correlation between the electronegativity of anion dopants and OER intrinsic activity in spinel oxides and provides a simple and effective method to modulate the electronic structure of spinel oxides by doping anions with different electronegativities, which can be a new avenue for the rational design of high-performance spinel electrocatalysts.

摘要
电子结构和电导率是析氧反应活性的重要描述符, 它们可以通过掺杂来调节. 鉴于金属掺杂通常会减少电催化剂的活性位点数量, 本工作探究了阴离子掺杂对尖晶石钴酸锌(ZCO)电子结构及其析氧活性的影响. 与三价钴为主的ZCO相比, 用电负性较低的硫取代氧会提高低自旋态(t2g6eg1)二价钴的占比, 其析氧活性要高于低自旋态的三价钴(t2g6eg0). 掺硫钴酸锌(ZCO-S)中钴离子和阴离子之间的电子密度的再分布导致了二价钴的增多, 而且钴和硫离子间的强共价作用也会加速电荷迁移. ZCO-S在1.65伏(相对于可逆氢电极)下的比活性比原始ZCO高11倍. 相反, 掺入具有较高电负性和价态的氟(F)并不能有效地改善电子结构, 最终导致材料析氧活性的降低. 本工作建立了所掺阴离子的电负性与钴酸锌本征析氧活性之间的联系, 并提供了一种通过掺杂不同电负性的阴离子来调控尖晶石氧化物的电子结构的简单有效的方法,这为合理设计高性能尖晶石电催化剂提供了新途径.
Similar content being viewed by others
References
Li X, Sun Y, Wu Q, et al. Optimized electronic configuration to improve the surface absorption and bulk conductivity for enhanced oxygen evolution reaction. J Am Chem Soc, 2019, 141: 3121–3128
Huang Y, Zhang SL, Lu XF, et al. Trimetallic spinel NiCo2−xFexO4 nanoboxes for highly efficient electrocatalytic oxygen evolution. Angew Chem Int Ed, 2021, 60: 11841–11846
Duan Y, Lee JY, Xi S, et al. Anodic oxidation enabled cation leaching for promoting surface reconstruction in water oxidation. Angew Chem Int Ed, 2021, 60: 7418–7425
Zhao Q, Yan Z, Chen C, et al. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem Rev, 2017, 117: 10121–10211
Xiao Z, Huang YC, Dong CL, et al. Operando identification of the dynamic behavior of oxygen vacancy-rich Co3O4 for oxygen evolution reaction. J Am Chem Soc, 2020, 142: 12087–12095
Sun S, Sun Y, Zhou Y, et al. Shifting oxygen charge towards octahedral metal: A way to promote water oxidation on cobalt spinel oxides. Angew Chem Int Ed, 2019, 58: 6042–6047
Wei C, Feng Z, Scherer GG, et al. Cations in octahedral sites: A descriptor for oxygen electrocatalysis on transition-metal spinels. Adv Mater, 2017, 29: 1606800
Suntivich J, May KJ, Gasteiger HA, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science, 2011, 334: 1383–1385
Zhu Y, Zhou W, Yu J, et al. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem Mater, 2016, 28: 1691–1697
Tong Y, Guo Y, Chen P, et al. Spin-state regulation of perovskite cobaltite to realize enhanced oxygen evolution activity. Chem, 2017, 3: 812–821
Duan Y, Sun S, Xi S, et al. Tailoring the Co 3d-O 2p covalency in LaCoO3 by Fe substitution to promote oxygen evolution reaction. Chem Mater, 2017, 29: 10534–10541
Li X, Cheng Z, Wang X. Understanding the mechanism of the oxygen evolution reaction with consideration of spin. Electrochem Energ Rev, 2020, 4: 136–145
Lee JG, Hwang J, Hwang HJ, et al. A new family of perovskite catalysts for oxygen-evolution reaction in alkaline media: BaNiO3 and BaNi0.83O2.5. J Am Chem Soc, 2016, 138: 3541–3547
Zhao B, Zhang L, Zhen D, et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat Commun, 2017, 8: 14586
Zhou Y, Sun S, Xi S, et al. Superexchange effects on oxygen reduction activity of edge-sharing [CoxMn1−xO6] octahedra in spinel oxide. Adv Mater, 2018, 30: 1705407
Guo Y, Tong Y, Chen P, et al. Engineering the electronic state of a perovskite electrocatalyst for synergistically enhanced oxygen evolution reaction. Adv Mater, 2015, 27: 5989–5994
Li H, Sun S, Xi S, et al. Metal-oxygen hybridization determined activity in spinel-based oxygen evolution catalysts: A case study of ZnFe2−xCrxO4. Chem Mater, 2018, 30: 6839–6848
Wang K, Wang Y, Yang B, et al. Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis. Energy Environ Sci, 2022, 15: 2356–2365
Li F, Han GF, Jeon JP, et al. Surface electronic modulation with heterosingle atoms to enhance oxygen evolution catalysis. ACS Nano, 2021, 15: 11891–11897
Zhou Y, Sun S, Wei C, et al. Significance of engineering the octahedral units to promote the oxygen evolution reaction of spinel oxides. Adv Mater, 2019, 31: 1902509
Duan Y, Sun S, Sun Y, et al. Mastering surface reconstruction of metastable spinel oxides for better water oxidation. Adv Mater, 2019, 31: 1807898
Wu T, Ren X, Sun Y, et al. Spin pinning effect to reconstructed oxyhydroxide layer on ferromagnetic oxides for enhanced water oxidation. Nat Commun, 2021, 12: 3634
Sun Y, Ren X, Sun S, et al. Engineering high-spin state cobalt cations in spinel zinc cobalt oxide for spin channel propagation and active site enhancement in water oxidation. Angew Chem Int Ed, 2021, 60: 14536–14544
Kim TW, Woo MA, Regis M, et al. Electrochemical synthesis of spinel type ZnCo2O4 electrodes for use as oxygen evolution reaction catalysts. J Phys Chem Lett, 2014, 5: 2370–2374
Xiang K, Wu D, Fan Y, et al. Enhancing bifunctional electrodes of oxygen vacancy abundant ZnCo2O4 nanosheets for supercapacitor and oxygen evolution. Chem Eng J, 2021, 425: 130583
Bao J, Wang Z, Liu W, et al. ZnCo2O4 ultrathin nanosheets towards the high performance of flexible supercapacitors and bifunctional electrocatalysis. J Alloys Compd, 2018, 764: 565–573
Menezes PW, Indra A, Bergmann A, et al. Uncovering the prominent role of metal ions in octahedral versus tetrahedral sites of cobalt-zinc oxide catalysts for efficient oxidation of water. J Mater Chem A, 2016, 4: 10014–10022
Zhang J, Zhang D, Yang Y, et al. Facile synthesis of ZnCo2O4 mesoporous structures with enhanced electrocatalytic oxygen evolution reaction properties. RSC Adv, 2016, 6: 92699–92704
Mai Z, Duan W, Wang K, et al. Integrating ZnCo2O4 submicro/nanospheres with CoxSey nanosheets for the oxygen evolution reaction and zinc-air batteries. Sustain Energy Fuels, 2020, 4: 2184–2191
Pan Y, Zeng W, Li L, et al. A facile synthesis of ZnCo2O4 nanocluster particles and the performance as anode materials for lithium ion batteries. Nano-Micro Lett, 2017, 9: 20
Hao S, Zhang B, Ball S, et al. Synthesis of multimodal porous ZnCo2O4 and its electrochemical properties as an anode material for lithium ion batteries. J Power Sources, 2015, 294: 112–119
Kathalingam A, Ramesh S, Yadav HM, et al. Nanosheet-like ZnCo2O4@nitrogen doped graphene oxide/polyaniline composite for supercapacitor application: Effect of polyaniline incorporation. J Alloys Compd, 2020, 830: 154734
Wei W, Chen W, Ivey DG. Rock salt-spinel structural transformation in anodically electrodeposited Mn-Co-O nanocrystals. Chem Mater, 2008, 20: 1941–1947
Xiao X, Wang G, Zhang M, et al. Electrochemical performance of mesoporous ZnCo2O4 nanosheets as an electrode material for supercapacitor. Ionics, 2018, 24: 2435–2443
Carbone M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J Electroanal Chem, 2018, 815: 151–157
Gao X, Liu J, Sun Y, et al. Optimized Co2+(Td)-O-Fe3+(Oh) electronic states in a spinel electrocatalyst for highly efficient oxygen evolution reaction performance. Inorg Chem Front, 2019, 6: 3295–3301
Chang S, Zhang H, Zhang Z. FeCo alloy/N, S dual-doped carbon composite as a high-performance bifunctional catalyst in an advanced rechargeable zinc-air battery. J Energy Chem, 2021, 56: 64–71
Zhang Z, Li J, Qian J, et al. Significant change of metal cations in geometric sites by magnetic-field annealing FeCo2O4 for enhanced oxygen catalytic activity. Small, 2022, 18: 2104248
Gao L, Chang S, Zhang Z. High-quality CoFeP nanocrystal/N, P dual-doped carbon composite as a novel bifunctional electrocatalyst for rechargeable Zn-air battery. ACS Appl Mater Interfaces, 2021, 13: 22282–22291
Huang XC, Zhang JY, Wu M, et al. Electronic structure and p-type conduction mechanism of spinel cobaltite oxide thin films. Phys Rev B, 2019, 100: 115301
Merz M, Nagel P, Pinta C, et al. X-ray absorption and magnetic circular dichroism of LaCoO3, La0.7Ce0.3CoO3, and La0.7Sr0.3CoO3 films: Evidence for cobalt-valence-dependent magnetism. Phys Rev B, 2010, 82: 174416
Abbate M, Fuggle JC, Fujimori A, et al. Electronic structure and spinstate transition of LaCoO3. Phys Rev B, 1993, 47: 16124–16130
Liu H, Li X, Peng C, et al. Activating the lattice oxygen in (Bi0.5Co0.5)2O3 by vacancy modulation for efficient electrochemical water oxidation. J Mater Chem A, 2020, 8: 13150–13159
Tang L, Fan T, Chen Z, et al. Binary-dopant promoted lattice oxygen participation in OER on cobaltate electrocatalyst. Chem Eng J, 2021, 417: 129324
Sun S, Li H, Xu ZJ. Impact of surface area in evaluation of catalyst activity. Joule, 2018, 2: 1024–1027
Wei C, Rao RR, Peng J, et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv Mater, 2019, 31: 1806296
Shamloofard M, Shahrokhian S, Amini MK. Mesoporous nanostructures of NiCo-LDH/ZnCo2O4 as an efficient electrocatalyst for oxygen evolution reaction. J Colloid Interface Sci, 2021, 604: 832–843
Liu J, Xie Y, Nan Y, et al. ZnCo2O4 nanoparticles derived from dual-metal-organic-frameworks embedded in multiwalled carbon nanotubes: A favorable electrocatalyst for the water splitting. Electrochim Acta, 2017, 257: 233–242
Cheng H, Su CY, Tan ZY, et al. Interacting ZnCo2O4 and Au nanodots on carbon nanotubes as highly efficient water oxidation electrocatalyst. J Power Sources, 2017, 357: 1–10
Xiong T, Tan Z, Mi Y, et al. On-site generated metal organic framework-deriving core/shell ZnCo2O4/ZnO nanoarray for better water oxidation. Nanotechnology, 2019, 30: 495405
Ma Y, Yang Y, Dai X, et al. Simultaneous modulation of composition and oxygen vacancies on hierarchical ZnCo2O4/Co3O4/NC-CNT mesoporous dodecahedron for enhanced oxygen evolution reaction. Chem Eur J, 2018, 24: 18689–18695
Zhang D, Wang Z, Li J, et al. MOF-derived ZnCo2O4 porous micro-rice with enhanced electro-catalytic activity for the oxygen evolution reaction and glucose oxidation. RSC Adv, 2020, 10: 9063–9069
Pan J, Wang F, Zhang L, et al. Clean synthesis of ZnCo2O4@ZnCo-LDHs yolk-shell nanospheres composed of ultra-thin nanosheets with enhanced electrocatalytic properties. Inorg Chem Front, 2019, 6: 220–225
Yu Z, Bai Y, Zhang N, et al. Metal-organic framework-derived heterostructured ZnCo2O4@FeOOH hollow polyhedrons for oxygen evolution reaction. J Alloys Compd, 2020, 832: 155067
Naveen MH, Bui TL, Lee L, et al. Nanostructuring matters: Stabilization of electrocatalytic oxygen evolution reaction activity of ZnCo2O4 by zinc leaching. ACS Appl Mater Interfaces, 2022, 14: 15165–15175
Yang H, Gao S, Rao D, et al. The regulation mechanism of cationic substitution in morphology-controlled oxy-spinel for oxygen evolution reaction. J Catal, 2022, 407: 221–231
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U2032154), the Key Research and Development Program of Anhui (202004a05020072), Anhui Initiative in Quantum Information Technologies (AHY100000), and Anhui Provincial Natural Science Foundation (1908085ME119).
Author information
Authors and Affiliations
Contributions
Lu Y, Fu Z, and Cheng Z conceived the project. Xiong B fabricated the samples. Ge L conducted the Bader charge analysis. Lei X and Li W performed the XAS characterization. Wang Y and Yang J performed the UV-vis experiments. Xiong B wrote the manuscript. Li X and Fu Z revised the manuscript. All the authors discussed the results and contributed to the manuscript preparation.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Bing Xiong received his BSc degree (2020) from Hefei University of Technology under the supervision of Prof. Yong Zhang. Now, he is a PhD candidate at the University of Science and Technology of China (USTC). His research mainly focuses on the modulation of electronic structure and magnetic property for energy conversion applications.
Zhengping Fu is an associate professor at the USTC. He graduated from the Department of Materials Science and Engineering, USTC in 1999 with a PhD degree. Then he has been working at the USTC. He did post-doctoral research at Tsing Hua University (Hsinchu) from 2003 to 2004. His research is on new functional materials and electrocatalysts.
Xiaoning Li is an associate research fellow at the Institute for Superconducting and Electronic Materials (ISEM), University of Wollongong. She obtained her PhD degree in material physics and chemistry from the University of Science and Technology of China in 2016. Currently, she is focusing on the correlation of crystal and electrontic structure, physical property, and the energy conversion efficiency of electrocatalysts for energy conversion applications.
Yalin Lu is the distinguished professor at the USTC and the director of Anhui Laboratory of Advanced Photon Science and Technology. He received a PhD degree in physics from Nanjing University in 1991. He was a professor at the Air Force Academy, Tufts University and Lawrence Berkeley National Laboratory. His research focuses on THz optics and materials, optoelectronics, materials physics of complex oxides, and materials for energy conversion.
Electronic Supplementary Material
40843_2022_2335_MOESM1_ESM.pdf
Tailoring the electronic structure of ZnCo2O4 by incorporating anions with low electronegativity to improve the water oxidation activity
Rights and permissions
About this article
Cite this article
Xiong, B., Ge, L., Lei, X. et al. Tailoring the electronic structure of ZnCo2O4 by incorporating anions with low electronegativity to improve the water oxidation activity. Sci. China Mater. 66, 1793–1800 (2023). https://doi.org/10.1007/s40843-022-2335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2335-1