Abstract
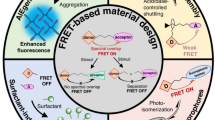
Stimuli-responsive aggregation-induced emission (AIE) materials with regulated color and fluorescence have shown promising applications in smart optical systems, while most of them are only regulated by a single stimulus. Herein, a cyanostilbene-based fluorophore (Z-BDPA) with AIE enhancement properties is elaborately designed, which exhibits multiple regulable color and fluorescence owing to its various photochemical reactions and reversible pH response. After exposure to 420 nm light, the green emission of Z-BDPA in tetrahydrofuran (THF) becomes brighter owing to the Z→E isomerization, which can be recovered under 365 nm irradiation; the yellow fluorescence of Z-BDPA in THF/H2O mixtures with 90% water fraction (fw) becomes stronger yellow-green fluorescence due to the photodimerization. At acidic conditions, Z-BDPA powder with yellow fluorescence can become another AIEgen Z-p-BDPA with blue fluorescence via protonation, which would be recovered through deprotonation under basic conditions. Under ultraviolet light irradiation, the blue fluorescence of Z-p-BDPA is blue-shifted and increased due to the photo-isomerization and subsequent photocyclodehydrogenation reactions. Based on the regulable color and fluorescence upon the photochemical and pH responses, Z-BDPA has been successfully applied in photo-patterning and anti-counterfeiting.

摘要
本文精心设计了一种具有聚集诱导发光增强特性的氰基苯乙烯 基荧光团(Z-BDPA), 由于其具有各种光化学反应和可逆的pH响应而呈现出多种可调节的颜色和荧光. 在420 nm光照射下, Z-BDPA在四氢呋喃(THF)中发生Z→E异构化, 其绿色荧光增强, 该过程在365 nm光照射下可恢复; 由于发生光二聚化过程, Z-BDPA在水含量(fw)为90%的THF/ H2O混合体系中的黄色荧光变为更强的黄绿色荧光. 在酸性条件下, 具有黄色荧光的Z-BDPA粉末通过质子化过程变为具有蓝色荧光的AIE荧光团Z-p-BDPA, 该过程可通过在碱性条件下的去质子化作用恢复. 在365 nm光照射下, Z-p-BDPA的蓝色荧光由于光异构化过程和随后的光环脱氢反应而蓝移且增强. 基于其对于光化学反应和pH响应而呈现出的可调控的颜色和荧光, Z-BDPA可成功应用于光图案化和防伪.
Similar content being viewed by others
References
Xu XY, Lian X, Hao JN, et al. A double-stimuli-responsive fluorescent center for monitoring of food spoilage based on dye covalently modified EuMOFs: From sensory hydrogels to logic devices. Adv Mater, 2017, 29:1702298
Tsang MK, Bai G, Hao J. Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chem Soc Rev, 2015, 44: 1585–1607
Mazrad ZAI, Lee K, Chae A, et al. Progress in internal/external stimuli responsive fluorescent carbon nanoparticles for theranostic and sensing applications. J Mater Chem B, 2018, 6: 1149–1178
Cheng Y, Dai J, Sun C, et al. An intracellular H2O2-responsive AIEgen for the peroxidase-mediated selective imaging and inhibition of inflammatory cells. Angew Chem Int Ed, 2018, 57: 3123–3127
Chen JF, Lin Q, Zhang YM, et al. Pillararene-based fluorescent chemosensors: Recent advances and perspectives. Chem Commun, 2017, 53: 13296–13311
Liu S, Cheng Y, Li Y, et al. Manipulating solid-state intramolecular motion toward controlled fluorescence patterns. ACS Nano, 2020, 14: 2090–2098
Huang G, Xia Q, Huang W, et al. Multiple anti-counterfeiting guarantees from a simple tetraphenylethylene derivative-high-contrasted and multi-state mechanochromism and photochromism. Angew Chem, 2019, 131: 17978–17983
Hong Y, Lam JWY, Tang BZ. Aggregation-induced emission. Chem Soc Rev, 2011, 40: 5361
Luo J, Xie Z, Lam JWY, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun, 2001, 1740–1741
Peng Q, Shuai Z. Molecular mechanism of aggregation-induced emission. Aggregate, 2021, 2: e91
Jia XR, Yu HJ, Chen J, et al. Stimuli-responsive properties of aggregation-induced-emission compounds containing a 9,10-distyrylanthracene moiety. Chem Eur J, 2018, 24: 19053–19059
Zhao J, Chi Z, Zhang Y, et al. Recent progress in the mechanofluorochromism of cyanoethylene derivatives with aggregation-induced emission. J Mater Chem C, 2018, 6: 6327–6353
Mei J, Leung NLC, Kwok RTK, et al. Aggregation-induced emission: Together we shine, united we soar! Chem Rev, 2015, 115: 11718–11940
Hu JJ, Jiang W, Yuan L, et al. Recent advances in stimuli-responsive theranostic systems with aggregation-induced emission characteristics. Aggregate, 2021, 2: 48–65
Li Q, Li Z. Molecular packing: Another key point for the performance of organic and polymeric optoelectronic materials. Acc Chem Res, 2020, 53: 962–973
Li Q, Li Z. Miracles of molecular uniting. Sci China Mater, 2020, 63: 177–184
Stoychev G, Kirillova A, Ionov L. Light-responsive shape-changing polymers. Adv Opt Mater, 2019, 7: 1900067
Gliozzi AS, Miniaci M, Chiappone A, et al. Tunable photo-responsive elastic metamaterials. Nat Commun, 2020, 11: 1–8
Luo W, Wang G. Photo-responsive fluorescent materials with aggregation-induced emission characteristics. Adv Opt Mater, 2020, 8: 2001362
Yan Q, Wang S. Fusion of aggregation-induced emission and photochromics for promising photoresponsive smart materials. Mater Chem Front, 2020, 4: 3153–3175
Li Y, Li Y, Feng Z, et al. An AIEgen-based luminescent photo-responsive system used as concealed anti-counterfeit material. J Lumin, 2019, 216: 116750
Yu Q, Su X, Zhang T, et al. Non-invasive fluorescence switch in polymer films based on spiropyran-photoacid modified TPE. J Mater Chem C, 2018, 6: 2113–2122
He Z, Shan L, Mei J, et al. Aggregation-induced emission and aggregation-promoted photochromism of bis(diphenylmethylene)dihydroacenes. Chem Sci, 2015, 6: 3538–3543
Wei P, Zhang JX, Zhao Z, et al. Multiple yet controllable photoswitching in a single AIEgen system. J Am Chem Soc, 2018, 140: 1966–1975
Gu X, Zhao E, Lam JWY, et al. Mitochondrion-specific live-cell bioprobe operated in a fluorescence turn-on manner and a well-designed photoactivatable mechanism. Adv Mater, 2015, 27: 7093–7100
Huang Q, Li W, Mao Z, et al. Dynamic molecular weaving in a two-dimensional hydrogen-bonded organic framework. Chem, 2021, 7: 1321–1332
Chen PZ, Zhang H, Niu LY, et al. A solid-state fluorescent material based on carbazole-containing difluoroboron β-diketonate: Multiple chromisms, the self-assembly behavior, and optical waveguides. Adv Funct Mater, 2017, 27: 1700332
Wu Z, Wang Q, Li P, et al. Photochromism of neutral spiropyran in the crystalline state at room temperature. J Mater Chem C, 2021, 9: 6290–6296
Lai Q, Liu Q, Zhao K, et al. Rational design and synthesis of yellow-light emitting triazole fluorophores with AIE and mechanochromic properties. Chem Commun, 2019, 55: 4603–4606
Zhang M, Li J, Yu L, et al. Tuning the fluorescence based on the combination of TICT and AIE emission of a tetraphenylethylene with D-π-A structure. RSC Adv, 2020, 10: 14520–14524
Zhu L, Yang C, Qin J. An aggregation-induced blue shift of emission and the self-assembly of nanoparticles from a novel amphiphilic oligofluorene. Chem Commun, 2008, 6303
Mazumdar P, Das D, Sahoo GP, et al. Aggregation induced emission enhancement of 4,4′-bis(diethylamino)benzophenone with an exceptionally large blue shift and its potential use as glucose sensor. Phys Chem Chem Phys, 2015, 17: 3343–3354
Lin S, Gutierrez-Cuevas KG, Zhang X, et al. Fluorescent photochromic α-cyanodiarylethene molecular switches: An emerging and promising class of functional diarylethene. Adv Funct Mater, 2021, 31: 2007957
Seo J, Chung JW, Kwon JE, et al. Photoisomerization-induced gel-to-sol transition and concomitant fluorescence switching in a transparent supramolecular gel of a cyanostilbene derivative. Chem Sci, 2014, 5: 4845–4850
Kim KY, Jung SH, Lee S, et al. Cyanostilbene-based supramolecular polymerization from one-dimensional to two-dimensional nanostructures via photoreactions. J Phys Chem C, 2018, 122: 22143–22149
Wu Y, Zhang S, Pei J, et al. Photochromic fluorescence switching in liquid crystalline polynorbornenes with α-cyanostilbene side-chains. J Mater Chem C, 2020, 8: 6461–6469
Martínez-Abadía M, Robles-Hernández B, de la Fuente MR, et al. Photoresponsive cyanostilbene bent-core liquid crystals as new materials with light-driven modulated polarization. Adv Mater, 2016, 28: 6586–6591
Zhang Z, Liu X, Feng Y, et al. Aggregation-mediated photo-responsive luminescence of cyanostilbene based cruciform AIEgens. J Mater Chem C, 2021, 9: 975–981
Martínez-Abadía M, Varghese S, Romero P, et al. Highly light-sensitive luminescent cyanostilbene flexible dimers. Adv Opt Mater, 2017, 5: 1600860
Wei P, Li Z, Zhang JX, et al. Molecular transmission: Visible and rate-controllable photoreactivity and synergy of aggregation-induced emission and host-guest assembly. Chem Mater, 2019, 31: 1092–1100
Wang Y, Tan X, Zhang YM, et al. Dynamic behavior of molecular switches in crystal under pressure and its reflection on tactile sensing. J Am Chem Soc, 2015, 137: 931–939
Hu W, He T, Zhao H, et al. Stimuli-responsive reversible switching of intersystem crossing in pure organic material for smart photodynamic therapy. Angew Chem Int Ed, 2019, 58: 11105–11111
Zhang D, Fan Y, Chen H, et al. CO2-activated reversible transition between polymersomes and micelles with AIE fluorescence. Angew Chem, 2019, 131: 10366–10371
Luo W, Wu B, Xu X, et al. A triple pH-responsive AIEgen: Synthesis, optical properties and applications. Chem Eng J, 2022, 431: 133717
Wang Z, Nie H, Yu Z, et al. Multiple stimuli-responsive and reversible fluorescence switches based on a diethylamino-functionalized tetraphenylethene. J Mater Chem C, 2015, 3: 9103–9111
Wang C, Li Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater Chem Front, 2017, 1: 2174–2194
Yang H, Li M, Li C, et al. Unraveling dual aggregation-induced emission behavior in steric-hindrance photochromic system for super resolution imaging. Angew Chem, 2020, 132: 8638–8648
Zhu L, Ang CY, Li X, et al. Luminescent color conversion on cyanostilbene-functionalized quantum dots via in-situ photo-tuning. Adv Mater, 2012, 24: 4020–4024
Li Z, Twieg RJ. Photocyclodehydrofluorination. Chem Eur J, 2015, 21: 15534–15539
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51373025) and the Program for New Century Excellent Talents in University (NCET-11-0582).
Author information
Authors and Affiliations
Contributions
Author contributions Luo W conducted all experiments and writing. Wang G conceived the project and revised the paper. All authors contributed to the general discussion.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper. Crystallographic data for the structural analyses of Z-BDPA have been deposited with the Cambridge Crystallographic Data Centre bearing the CCDC Nos. 2168298 (deposit@ccdc.cam.ac.uk).
Weihua Luo received her MSc degree from the Northwest University in 2018. Now she is studying for her PhD degree in Prof. Wang’s group at the University of Science and Technology Beijing. Her research interest focuses on the construction and applications of stimuli-responsive fluorescent materials with AIE characteristics.
Guojie Wang received his PhD degree in polymer chemistry and physics from Jilin University in 2000. Then he worked as a post-doctor at Tsinghua University. In 2002, he joined the Institute of Chemistry, Chinese Academy of Sciences. In 2005, he joined Georgia Institute of Technology. In 2006, he moved to Katholieke Universiteit Leuven. In 2008, he joined the University of Science and Technology Beijing. His research interest focuses on photoswitches and fluorescent materials.
Supporting Information
Rights and permissions
About this article
Cite this article
Luo, W., Xu, X., Tang, Y. et al. Multiregulated color and fluorescence of a cyanostilbene-based AIEgen by light and pH. Sci. China Mater. 66, 1180–1188 (2023). https://doi.org/10.1007/s40843-022-2215-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2215-1