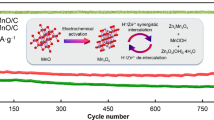
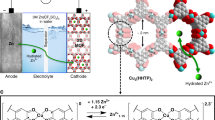
Abstract
Ion intercalation is an effective strategy for improving the cycle stability and rate performance of δ-MnO2 as a cathode material for aqueous zinc-ion batteries. However, in practice, ion selection appears rather arbitrary. In this work, Cu2+ was chosen for δ-MnO2 intercalation because although Cu2+ and Zn2+ have similar diameters, Cu2+ has a slightly higher electronegativity (1.359) than Zn2+ (1.347). Therefore, Cu2+ has a stronger interaction with the MnO2 lattice than Zn2+ and can be stable during the intercalation/deintercalation of Zn2+ and H+. Results showed that the performance of Cu-doped δ-MnO2 (CMO) was greatly improved. Moreover, at the high current density of 2 A g−1, CMO achieved excellent cycle stability with 100% capacity retention after 600 cycles, whereas pristine δ-MnO2 exhibited only 23% capacity retention. When the current density was increased from 0.2 to 2.0 A g−1, the CMO electrode also delivered remarkable rate performance with 72% capacity retention, which was considerably higher than the 32% capacity retention demonstrated by pristine δ-MnO2. Given that Cu2+ has a greater electronegativity than Zn2+, the Cu-O bond formed in CMO acted as a stable structural column and greatly improved the stability of CMO. Cu2+ doping also increased the electronic conductivity and ionic conductivity of CMO and reduced the charge transfer resistance of H+ and Zn2+ at the electrode/electrolyte interface, which improved the rate performance of CMO greatly. This work provides new insights into intercalation strategies to improve the electrochemical performance of batteries.

摘要
离子插层已成为提高δ-MnO2作为水系锌离子电池正极材料的循环稳定性和倍率性能的有效策略, 但在实践中离子的选择似乎相当随意. 本工作选择Cu2+插层δ-MnO2, 因为Cu2+和Zn2+具有相似的直径, 但Cu2+的电负性(1.359)略高于Zn2+(1.347). 因此, Cu2+与MnO2晶格具有更强的相互作用, 并且在Zn2+和H+的嵌入/脱出循环期间可保持稳定. Cu掺杂的δ-MnO2(CMO)生成了Cu–O键, 其电化学性能得到了较大的改善. 在2 A g−1的高电流密度下循环600次后, CMO表现出出色的循环稳定性和100%的容量保持率, 而原始δ-MnO2的容量保持率仅为23%. 当电流密度从0.2增加到2.0 A g−1时, CMO还表现出优异的倍率性能, 容量保持率为72%, 远高于原始δ-MnO2(32%). 由于Cu2+比Zn2+具有更大的电负性, 因此Cu–O键作为稳定的“结构之柱”提高了CMO的循环稳定性. Cu2+掺杂还提高了CMO的电子电导率和离子电导率, 降低了H+和Zn2+在电极/电解质界面的电荷转移电阻, 从而提高了其倍率性能. 这项工作为使用插层策略提高电池电化学性能提供了新的见解.
Similar content being viewed by others
References
Zhao J, Wu W, Jia X, et al. High-value utilization of biomass waste: From garbage floating on the ocean to high-performance rechargeable Zn-MnO2 batteries with superior safety. J Mater Chem A, 2020, 8: 18198–18206
Yuan F, Gao G, Jiang X, et al. Suppressing the metal-metal interaction by CoZn0.5V1.5O4 derived from two-dimensional metal-organic frameworks for supercapacitors. Sci China Mater, 2021, 65: 105–114
Cao J, Xie Y, Yang Y, et al. Achieving uniform Li plating/stripping at ultrahigh currents and capacities by optimizing 3D nucleation sites and Li2Se-enriched SEI. Adv Sci, 2022, 9: 2104689
Xie Y, Cao J, Wang X, et al. MOF-derived bifunctional Co0.85Se nanoparticles embedded in N-doped carbon nanosheet arrays as efficient sulfur hosts for lithium-sulfur batteries. Nano Lett, 2021, 21: 8579–8586
Jiang S, Yun S, Cao H, et al. Porous carbon matrix-encapsulated MnO in situ derived from metal-organic frameworks as advanced anode materials for Li-ion capacitors. Sci China Mater, 2021, 65: 59–68
Liu Q, Wang L, Liu X, et al. N-doped carbon-coated Co3O4 nanosheet array/carbon cloth for stable rechargeable Zn-air batteries. Sci China Mater, 2018, 62: 624–632
Wei T, Peng Y, Mo L, et al. Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries. Sci China Mater, 2022, 65: 1156–1164
Zhou X, Zhang Q, Hao Z, et al. Unlocking the allometric growth and dissolution of Zn anodes at initial nucleation and an early stage with atomic force microscopy. ACS Appl Mater Interfaces, 2021, 13: 53227–53234
Liu L, Yang W, Chen H, et al. High zinc-ion intercalation reaction activity of MoS2 cathode based on regulation of thermodynamic metastability and interlayer water. Electrochim Acta, 2022, 410: 140016
Cui F, Hu F, Yu X, et al. In-situ tuning the NH4+ extraction in (NH4)2V4O9 nanosheets towards high performance aqueous zinc ion batteries. J Power Sources, 2021, 492: 229629
Zhang Y, Wang Y, Lu L, et al. Vanadium hexacyanoferrate with two redox active sites as cathode material for aqueous Zn-ion batteries. J Power Sources, 2021, 484: 229263
Wang L, Huang KW, Chen J, et al. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci Adv, 2019, 5: eaax4279
Hao J, Long J, Li B, et al. Toward high-performance hybrid Zn-based batteries via deeply understanding their mechanism and using electrolyte additive. Adv Funct Mater, 2019, 29: 1903605
Liu Y, Wen Y, Zhang Y, et al. Reduced CoNi2S4 nanosheets decorated by sulfur vacancies with enhanced electrochemical performance for asymmetric supercapacitors. Sci China Mater, 2020, 63: 1216–1226
Wang L, Cao X, Xu L, et al. Transformed akhtenskite MnO2 from Mn3O4 as cathode for a rechargeable aqueous zinc ion battery. ACS Sustain Chem Eng, 2018, 6: 16055–16063
Hu K, Guan X, Lv R, et al. Stabilizing zinc metal anodes by artificial solid electrolyte interphase through a surface ion-exchanging strategy. Chem Eng J, 2020, 396: 125363
Park IJ, Choi SR, Kim JG. Aluminum anode for aluminum-air battery—Part II: Influence of In addition on the electrochemical characteristics of Al-Zn alloy in alkaline solution. J Power Sources, 2017, 357: 47–55
Thenuwara AC, Shumlas SL, Attanayake NH, et al. Copper-intercalated birnessite as a water oxidation catalyst. Langmuir, 2015, 31: 12807–12813
Li C, Xie X, Liu H, et al. Integrated ‘all-in-one’ strategy to stabilize zinc anodes for high-performance zinc-ion batteries. Natl Sci Rev, 2022, 9: nwab177
Wu Y, Tao Y, Zhang X, et al. Self-assembled α-MnO2 urchin-like microspheres as a high-performance cathode for aqueous Zn-ion batteries. Sci China Mater, 2020, 63: 1196–1204
Huang Y, Li Z, Jin S, et al. Carbon nanohorns/nanotubes: An effective binary conductive additive in the cathode of high energy-density zinc-ion rechargeable batteries. Carbon, 2020, 167: 431–438
Zhao Q, Song A, Ding S, et al. Preintercalation strategy in manganese oxides for electrochemical energy storage: Review and prospects. Adv Mater, 2020, 32: 2002450
Chao D, Zhou W, Ye C, et al. An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew Chem Int Ed, 2019, 58: 7823–7828
Jiao Y, Kang L, Berry-Gair J, et al. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J Mater Chem A, 2020, 8: 22075–22082
Du Z, Yu P, Wang L, et al. Cubic imidazolate frameworks-derived CoFe alloy nanoparticles-embedded N-doped graphitic carbon for discharging reaction of Zn-air battery. Sci China Mater, 2020, 63: 327–338
Yang F, Shen Y, Cen Z, et al. In situ construction of heterostructured bimetallic sulfide/phosphide with rich interfaces for high-performance aqueous Zn-ion batteries. Sci China Mater, 2021, 65: 356–363
Zhang B, Qin L, Fang Y, et al. Tuning Zn2+ coordination tunnel by hierarchical gel electrolyte for dendrite-free zinc anode. Sci Bull, 2022, 67: 955–962
Wang M, Yagi S. Layered birnessite MnO2 with enlarged interlayer spacing for fast Mg-ion storage. J Alloys Compd, 2020, 820: 153135
Joseph J, Nerkar J, Tang C, et al. Reversible intercalation of multivalent Al3+ ions into potassium-rich cryptomelane nanowires for aqueous rechargeable Al-ion batteries. ChemSusChem, 2019, 12: 3753–3760
Gao P, Metz P, Hey T, et al. The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets. Nat Commun, 2017, 8: 14559
Sun T, Nian Q, Zheng S, et al. Layered Ca0.28MnO2-0.5H2O as a high performance cathode for aqueous zinc-ion battery. Small, 2020, 16: 2000597
Liu Y, Qiao Y, Zhang W, et al. Nanostructured alkali cation incorporated δ-MnO2 cathode materials for aqueous sodium-ion batteries J Mater Chem A, 2015, 3: 7780–7785
Xu J, Hu X, Alam MA, et al. Al-doped δ-MnO2 coated by lignin for high-performance rechargeable aqueous zinc-ion batteries RSC Adv, 2021, 11: 35280–35286
Lin MX, Shao F, Weng S, et al. Boosted charge transfer in oxygen vacancy-rich K+ birnessite MnO2 for water oxidation and zinc-ion batteries. Electrochim Acta, 2021, 378: 138147
Wang S, Yuan Z, Zhang X, et al. Non-metal ion Co-insertion chemistry in aqueous Zn/MnO2 batteries. Angew Chem Int Ed, 2021, 60: 7056–7060
Singh J, Mishra V. Simultaneous removal of Cu2+, Ni2+ and Zn2+ ions using leftover Azadirachta indica twig ash. Bioremediation J, 2021, 25: 48–71
Guo S, Fang G, Liang S, et al. Structural perspective on revealing energy storage behaviors of silver vanadate cathodes in aqueous zinc-ion batteries. Acta Mater, 2019, 180: 51–59
Xu P, Wang C, Zhao B, et al. A high-strength and ultra-stable halloysite nanotubes-crosslinked polyacrylamide hydrogel electrolyte for flexible zinc-ion batteries. J Power Sources, 2021, 506: 230196
Zhang X, Wang L, Fu H. Recent advances in rechargeable Zn-based batteries. J Power Sources, 2021, 493: 229677
Wei C, Xu C, Li B, et al. Anomalous effect of K ions on electrochemical capacitance of amorphous MnO2. J Power Sources, 2013, 234: 1–7
Yadav GG, Wei X, Huang J, et al. A conversion-based highly energy dense Cu2+ intercalated Bi-birnessite/Zn alkaline battery. J Mater Chem A, 2017, 5: 15845–15854
Long J, Yang F, Cuan J, et al. Boosted charge transfer in twinborn α-(Mn2O3-MnO2) heterostructures: Toward high-rate and ultralong-life zinc-ion batteries. ACS Appl Mater Interfaces, 2020, 12: 32526–32535
Liu C, Tian M, Wang M, et al. Catalyzing zinc-ion intercalation in hydrated vanadates for aqueous zinc-ion batteries. J Mater Chem A, 2020, 8: 7713–7723
Guo C, Liu H, Li J, et al. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim Acta, 2019, 304: 370–377
Wang J, Wang JG, Liu H, et al. Zinc ion stabilized MnO2 nanospheres for high capacity and long lifespan aqueous zinc-ion batteries. J Mater Chem A, 2019, 7: 13727–13735
Li Q, Yin L, Li Z, et al. Copper doped hollow structured manganese oxide mesocrystals with controlled phase structure and morphology as anode materials for lithium ion battery with improved electrochemical performance. ACS Appl Mater Interfaces, 2013, 5: 10975–10984
Fang G, Zhu C, Chen M, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv Funct Mater, 2019, 29: 1808375
Ji J, Wan H, Zhang B, et al. Co2+/3+/4+-regulated electron state of Mn-O for superb aqueous zinc-manganese oxide batteries. Adv Energy Mater, 2021, 11: 2003203
Jabeen N, Xia Q, Savilov SV, et al. Enhanced pseudocapacitive performance of α-MnO2 by cation preinsertion. ACS Appl Mater Interfaces, 2016, 8: 33732–33740
Zhang Y, Liu Y, Liu Z, et al. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J Energy Chem, 2022, 64: 23–32
Chodankar NR, Dubal DP, Gund GS, et al. Flexible all-solid-state MnO2 thin films based symmetric supercapacitors. Electrochim Acta, 2015, 165: 338–347
Fenta FW, Olbasa BW, Tsai MC, et al. Electrochemical transformation reaction of Cu-MnO in aqueous rechargeable zinc-ion batteries for high performance and long cycle life. J Mater Chem A, 2020, 8: 17595–17607
Wang JG, Yang Y, Huang ZH, et al. Shape-controlled synthesis of hierarchical hollow urchin-shape α-MnO2 nanostructures and their electrochemical properties. Mater Chem Phys, 2013, 140: 643–650
Wang D, Wang L, Liang G, et al. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano, 2019, 13: 10643–10652
Jiang Y, Ba D, Li Y, et al. Noninterference revealing of “layered to layered” zinc storage mechanism of δ-MnO2 toward neutral Zn-Mn batteries with superior performance. Adv Sci, 2020, 7: 1902795
McKendry IG, Mohamad LJ, Thenuwara AC, et al. Synergistic in-layer cobalt doping and interlayer iron intercalation into layered MnO2 produces an efficient water oxidation electrocatalyst. ACS Energy Lett, 2018, 3: 2280–2285
Hu P, Zhu T, Wang X, et al. Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery. Nano Lett, 2018, 18: 1758–1763
Hu P, Zhu T, Ma J, et al. Porous V2O5 microspheres: A high-capacity cathode material for aqueous zinc-ion batteries. Chem Commun, 2019, 55: 8486–8489
Yan M, He P, Chen Y, et al. Water-lubricated intercalation in V2O5-nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv Mater, 2018, 30: 1703725
Yang W, Wang Y, Yang W, et al. Surface in situ doping modification over Mn2O3 for toluene and propene catalytic oxidation: The effect of isolated Cuδ+ insertion into the mezzanine of surface MnO2 cladding. ACS Appl Mater Interfaces, 2021, 13: 2753–2764
Selim AQ, Sellaoui L, Ahmed SA, et al. Statistical physics-based analysis of the adsorption of Cu2+ and Zn2+ onto synthetic cancrinite in single-compound and binary systems. J Environ Chem Eng, 2019, 7: 103217
Fan X, Liu H, Anang E, et al. Effects of electronegativity and hydration energy on the selective adsorption of heavy metal ions by synthetic NaX zeolite. Materials, 2021, 14: 4066
Zhu S, Huo W, Liu X, et al. Birnessite based nanostructures for supercapacitors: Challenges, strategies and prospects. Nanoscale Adv, 2020, 2: 37–54
Sun W, Wang F, Hou S, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J Am Chem Soc, 2017, 139: 9775–9778
Acknowledgements
This work was supported by Gansu Provincial Natural Science Foundation of China (17JR5RA198 and 21JR7RA470), the Cooperation Project of Gansu Academy of Sciences (2020HZ-2), the Fundamental Research Funds for the Central Universities (lzujbky-2018-119, lzujbky-2018-ct08, and lzujbky-2019-it23), and the Key Areas Scientific and Technological Research Projects in Xinjiang Production and Construction Corps (2018AB004), Hubei University of Arts and Science (2020kypytd002), and Xiangyang Science and Technology Research and Development (2020YL09).
Author information
Authors and Affiliations
Contributions
Author contributions Liu Z and Liu Y designed and engineered the samples; Liu Z and Zhang Y conceived the post-fabrication tuning of random modes; Liu Z and Liu X performed the experiments; Liu Z, Liu Y, Yan D, Huang J, and Peng S contributed to the theoretical analysis. All authors contributed to the general discussion.
Corresponding authors
Additional information
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information Supporting data are available in the online version of the paper.
Zhenhua Liu is currently a master student at the School of Materials and Energy, Lanzhou University. Her research interest focuses on aqueous zinc-ion batteries.
Shanglong Peng is a professor and doctoral advisor at Lanzhou University. He received his doctor’s degree from Lanzhou University in 2008. He was employed as an associate professor and professor at Lanzhou University. Currently, he mainly focuses on the relevant theoretical and technological innovations and applications of clean energy materials and devices, such as solar cells, lithium/ sodium-ion batteries, and photovoltaic coupled electrolytic water hydrogen production materials and devices related to hydrogen energy development.
Supporting Information
40843_2022_2179_MOESM1_ESM.pdf
Selection of Cu2+ for intercalation from the electronegativity perspective: Improving the cycle stability and rate performance of δ-MnO2 cathode material for aqueous zinc-ion batteries
Rights and permissions
About this article
Cite this article
Liu, Z., Liu, Y., Zhang, Y. et al. Selection of Cu2+ for intercalation from the electronegativity perspective: Improving the cycle stability and rate performance of δ-MnO2 cathode material for aqueous zinc-ion batteries. Sci. China Mater. 66, 531–540 (2023). https://doi.org/10.1007/s40843-022-2179-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2179-7