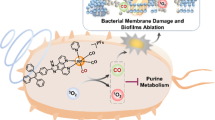
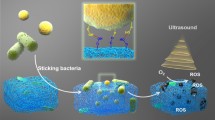
Abstract
Antibiotic is one of the greatest discoveries in human history. It has drastically promoted modern medicine and extended the average human lifespan. However, antibiotic resistance has become a global crisis today and the development of novel antibiotics is highly demanded. The traditional antibiotics not only kill the pathogen but also damage the resident microbiome in the human body, thus promoting antibiotic resistance and elevating the risk of patients for new infection. Here, we fabricated an activable metal-phenolic network nano-antibiotics (PEG-P18-Ag NPs) that can be selectively activated on the site of infection, thus presumably avoiding their impacts on the resident microbiome. We showed that PEG-P18-Ag NPs per se do not have any antibacterial activity. However, upon activation by the ultrasound, they triggered the generation of reactive oxygen species. Consequently, PEG-P18-Ag NPs remarkably killed various multi-drug resistant bacteria and established biofilms in vitro and in vivo. By RNA sequencing, we revealed that activated PEG-P18-Ag NPs produced a profound damaging effect on the bacteria. Collectively, we provided a novel approach for the new generation of antibiotics that selectively target infected bacteria.

摘要
抗生素的滥用引发了漫延全球的抗生素耐药危机. 多重耐药细 菌的出现对人类生命健康构成了极大的威胁. 此外, 传统抗生素在体内 杀死病原菌的同时, 对人体正常微生物菌群的破坏也增加了导致其他 感染和疾病的风险. 因此, 新型抗生素的研发显得日益紧迫. 在本文中, 我们合成了一种超声激活的金属多酚配位纳米抗生素(PEG-P18-Ag NPs), 其可在感染部位被选择性激活, 从而避免了对体内正常微生 物群体的影响. 实验证实PEG-P18-Ag NPs在未激活时不具有抗菌活性. 经超声激活后, 其可引起大量活性氧物种(ROS)的产生, 从而杀死多种 多重耐药菌及生物膜. 通过RNA测序技术, 本文进一步证实PEG-P18-Ag NPs对细菌细胞的细胞膜、核糖体、染色质及鞭毛等结构造成了严 重损伤. 总之, 本研究合成了一种靶向体内耐药菌的新型抗生素, 可实 现广谱、快速和可控的抗多重耐药菌治疗.
Similar content being viewed by others
References
David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature, 2014, 505: 559–563
Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature, 2011, 474: 327–336
Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell, 2016, 167: 915–932
Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun, 2018, 9: 141
Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. Br Med J, 2018, 361: k2179
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA, 2011, 108: 4554–4561
Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest, 2014, 124: 4212–4218
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med, 2015, 372: 1539–1548
Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature, 2012, 488: 621–626
Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med, 2016, 8: 343ra382
Korpela K, Salonen A, Virta LJ, et al. Intestinal microbiome is related to lifetime antibiotic use in finnish pre-school children. Nat Commun, 2016, 7: 10410
Turta O, Rautava S. Antibiotics, obesity and the link to microbes: What are we doing to our children? BMC Med, 2016, 14: 57
Gibson MK, Wang B, Ahmadi S, et al. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol, 2016, 1: 1602
Anthony WE, Burnham CAD, Dantas G, et al. The gut microbiome as a reservoir for antimicrobial resistance. J Infect Dis, 2021, 223: S209–S213
Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol, 2015, 27: 51–56
Ross JE, Flamm RK, Jones RN. Initial broth microdilution quality control guidelines for debio 1452, a Fabi inhibitor antimicrobial agent. Antimicrob Agents Chemother, 2015, 59: 7151–7152
Dickey SW, Cheung GYC, Otto M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov, 2017, 16: 457–471
Mijnendonckx K, Leys N, Mahillon J, et al. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals, 2013, 26: 609–621
Jung WK, Koo HC, Kim KW, et al. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol, 2008, 74: 2171–2178
Morones-Ramirez JR, Winkler JA, Spina CS, et al. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med, 2013, 5: 190ra181
Yakabe Y, Sano T, Ushio H, et al. Kinetic studies of the interaction between silver ion and deoxyribonucleic acid. Chem Lett, 1980, 9: 373–376
Möhler JS, Sim W, Blaskovich MAT, et al. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol Adv, 2018, 36: 1391–1411
Lansdown AB. Silver in health care: Antimicrobial effects and safety in use. Curr Probl Dermatol, 2006, 33: 17–34
Chernousova S, Epple M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew Chem Int Ed, 2013, 52: 1636–1653
Wei L, Lu J, Xu H, et al. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov Today, 2015, 20: 595–601
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, et al. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol, 2011, 9: 30
Siddiqi KS, Husen A. Fabrication of metal nanoparticles from fungi and metal salts: Scope and application. Nanoscale Res Lett, 2016, 11: 98
Siddiqi KS, Husen A. Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res Lett, 2016, 11: 363
Bruna T, Maldonado-Bravo F, Jara P, et al. Silver nanoparticles and their antibacterial applications. Int J Mol Sci, 2021, 22: 7202
Guo Y, Sun Q, Wu FG, et al. Polyphenol-containing nanoparticles: Synthesis, properties, and therapeutic delivery. Adv Mater, 2021, 33: 2007356
Chen Z, Farag MA, Zhong Z, et al. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv Drug Deliv Rev, 2021, 176: 113870
Son S, Kim JH, Wang X, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev, 2020, 49: 3244–3261
Liang S, Deng X, Ma P, et al. Recent advances in nanomaterial-assisted combinational sonodynamic cancer therapy. Adv Mater, 2020, 32: 2003214
Xu M, Zhou L, Zheng L, et al. Sonodynamic therapy-derived multimodal synergistic cancer therapy. Cancer Lett, 2021, 497: 229–242
Pavlíčková V, Škubník J, Jurášek M, et al. Advances in Purpurin 18 research: On cancer therapy. Appl Sci, 2021, 11: 2254
Zheng J, Tung SL, Leung KY. Regulation of a type III and a putative secretion system in Edwardsiella tarda by EsrC Is under the control of a two-component system, EsrA-EsrB. Infect Immun, 2005, 73: 4127–4137
Philippe N, Alcaraz JP, Coursange E, et al. Improvement of pcvd442, a suicide plasmid for gene allele exchange in bacteria. Plasmid, 2004, 51: 246–255
Ji X, Zou J, Peng H, et al. Alarmone Ap4A is elevated by aminoglycoside antibiotics and enhances their bactericidal activity. Proc Natl Acad Sci USA, 2019, 116: 9578–9585
Yan J, Bassler BL. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe, 2019, 26: 15–21
Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother, 2001, 45: 999–1007
Park HJ, Kim JY, Kim J, et al. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res, 2009, 43: 1027–1032
Repine JE, Fox RB, Berger EM. Hydrogen peroxide kills staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem, 1981, 256: 7094–7096
Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science, 1988, 240: 640–642
Kohanski MA, Dwyer DJ, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 2007, 130: 797–810
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol, 2010, 2: a000414
Lugtenberg B. Composition and function of the outer membrane of Escherichia coli. Trends Biochem Sci, 1981, 6: 262–266
Haiko J, Westerlund-Wikström B. The role of the bacterial flagellum in adhesion and virulence. Biology, 2013, 2: 1242–1267
Liu R, Ochman H. Stepwise formation of the bacterial flagellar system. Proc Natl Acad Sci USA, 2007, 104: 7116–7121
Smith BT, Walker GC. Mutagenesis and more: Umudc and the Escherichia coli sos response. Genetics, 1998, 148: 1599–1610
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res, 2008, 18: 85–98
Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radical Biol Med, 2014, 66: 75–87
Smirnova GV, Muzyka NG, Oktyabrsky ON. The role of antioxidant enzymes in response of Escherichia coli to osmotic upshift. FEMS Microbiol Lett, 2000, 186: 209–213
Staerck C, Gastebois A, Vandeputte P, et al. Microbial antioxidant defense enzymes. Microb Pathog, 2017, 110: 56–65
Acknowledgements
This study was supported by the National Natural Science Foundation of China (32171318 and 32101069), University of Macau (MYRG2019-000050-FHS and MYRG2020-00046-FHS), the Science and Technology Development Fund, Macau SAR (0109/2018/A3, 0058/2018/A2, 0113/2019/A2, 0103/2021/A and 0002/2021/AKP), and Shenzhen Science and Technology Innovation Commission, Shenzhen-Hong Kong-Macau Science and Technology Plan C (SGDX20201103093600004). We are thankful to the kind assistance and support from the Animal Research Core and Biological Imaging and Stem Cell Core, Faculty of Health Sciences, University of Macau.
Author information
Authors and Affiliations
Contributions
Lu C and Tian Y designed and carried out the experiments. Lu C took the lead in performing the experiments, analyzing the data and writing the manuscript with input from all authors. Tian Y synthesized the nanoparticles and evaluated the characterizations of nanoparticles. Tian H assisted in the nanoparticle synthesis. Li B helped with funding acquisition. Peng B provided several bacteria strains. Zheng J conceived the presented idea and assisted in the funding and supervising of the project. Dai Y conceived the study and was in charge of overall direction and planning. All authors discussed the results and commented on the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Chang Lu received her Master’s degree from the School of Laboratory Medicine, Chongqing Medical University, China. Now she is pursuing her PhD degree at the Faculty of Health and Sciences, University of Macau. Her research mainly focuses on the development of novel antibacterial compounds and materials for controlling bacterial antibiotic persistence and resistance.
Ye Tian received her BD degree (2017) and MD degree (2020) from the School of Public Health, Xiamen University in China. From August 2020 to now, she is a PhD student at the Faculty of Health Sciences, University of Macau. Her research mainly focuses on the synthesis and application of nanomedicines.
Jun Zheng is an associate professor at the Faculty of Health Sciences, University of Macau. He received his PhD degree from the Department of Biological Sciences, National University of Singapore. Then he joined Prof. John Mekalanos’ laboratory at the Department of Microbiology and Molecular Genetics, Harvard Medical School as a postdoctoral fellow. His research focuses on the mechanism of bacterial death, bacterial pathogenesis, and the development of new antibacterial agents.
Yunlu Dai is an assistant professor at the Faculty of Health Sciences, University of Macau. He received his PhD degree in 2014 from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. He then moved to the University of Melbourne as a research fellow. In 2016, he worked as a postdoctoral fellow at the National Institutes of Health. He initiated his independent research program in 2018 at the University of Macau. His research focuses on the multifunctional hybrid nanomaterials for biomedical application.
Electronic Supplementary Materials
Rights and permissions
About this article
Cite this article
Lu, C., Tian, Y., Tian, H. et al. An ultrasound activable metal-phenolic network nano-antibiotics for in vivo on-site infection therapy. Sci. China Mater. 66, 395–406 (2023). https://doi.org/10.1007/s40843-022-2125-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2125-1