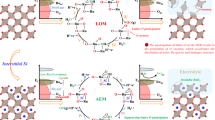
Abstract
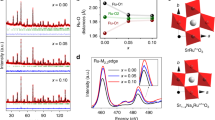
The design of highly active and stable catalysts for the oxygen evolution reaction (OER) in acidic media has become an attractive research area for the development of energy conversion and storage technologies. However, progress in this area has been limited by the poor understanding of the dynamic active structure of catalysts under realistic OER conditions. Here, an atomic Co-doped nanoporous RuO2 electrocatalyst, which exhibited excellent OER activity and stability in acidic conditions, was synthesized through annealing and etching of a nanoporous Co-Ru alloy. Operando X-ray absorption spectroscopy results confirmed that the etching strategy produced abundant oxygen vacancies around the metal centers in the atomic Co-doped nanoporous RuO2 electrocatalyst. These vacancies created contracted metal-oxygen ligand bonds under realistic OER conditions. The dynamic structural evolution of the synthesized electrocatalyst allowed them to experience lower kinetic barriers during OER catalysis, resulting in enhanced catalytic activity and stability. This study also provided atomic details on the active structure of the electrocatalyst and the influence of their structural evolution on OER activity.

摘要
设计在酸性介质中具有高活性和高稳定性的氧析出反应(OER) 催化剂对能量转换和储存技术的发展具有重要意义. 然而, 在实际OER 条件下催化剂的原子结构会发生变化, 且目前对其动态活性结构的认 识仍然不足. 本文中, 我们通过退火和蚀刻纳米多孔Co-Ru合金合成了 具有优异酸性OER活性和稳定性的原子级Co掺杂纳米多孔RuO2催化 剂. 原位X射线吸收光谱证实: 蚀刻策略可以在原子级Co掺杂纳米多孔 RuO2的金属中心周围产生丰富的氧空位, 从而在实际OER条件下产生 收缩的金属-氧配体键. 这种动态结构演变降低了催化活性位点在OER 过程中的动力学势垒, 因而催化剂的催化活性和稳定性大幅提高. 本研 究结果揭示了催化剂活性结构的原子细节以及它们的结构演化对催化 活性的影响.
Similar content being viewed by others
References
Seh ZW, Kibsgaard J, Dickens CF, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science, 2017, 355: eaad4998
Seitz LC, Dickens CF, Nishio K, et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science, 2016, 353: 1011–1014
Chatti M, Gardiner JL, Fournier M, et al. Intrinsically stable in situ generated electrocatalyst for long-term oxidation of acidic water at up to 80°C. Nat Catal, 2019, 2: 457–465
Wang J, Han L, Huang B, et al. Amorphization activated ruthenium-tellurium nanorods for efficient water splitting. Nat Commun, 2019, 10: 5692
Yang J, Ji Y, Shao Q, et al. A universal strategy to metal wavy nanowires for efficient electrochemical water splitting at pH-universal conditions. Adv Funct Mater, 2018, 28: 1803722
Lin Y, Tian Z, Zhang L, et al. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat Commun, 2019, 10: 162
Shan J, Guo C, Zhu Y, et al. Charge-redistribution-enhanced nanocrystalline Ru@IrOx electrocatalysts for oxygen evolution in acidic media. Chem, 2019, 5: 445–459
Kim JS, Kim B, Kim H, et al. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv Energy Mater, 2018, 8: 1702774
Zhao ZL, Wang Q, Huang X, et al. Boosting the oxygen evolution reaction using defect-rich ultra-thin ruthenium oxide nanosheets in acidic media. Energy Environ Sci, 2020, 13: 5143–5151
Zhao Y, Luo M, Chu S, et al. 3D nanoporous iridium-based alloy microwires for efficient oxygen evolution in acidic media. Nano Energy, 2019, 59: 146–153
Bhowmik T, Kundu MK, Barman S. Growth of one-dimensional RuO2 nanowires on g-carbon nitride: An active and stable bifunctional electrocatalyst for hydrogen and oxygen evolution reactions at all pH values. ACS Appl Mater Interfaces, 2016, 8: 28678–28688
DeSario PA, Chervin CN, Nelson ES, et al. Competitive oxygen evolution in acid electrolyte catalyzed at technologically relevant electrodes painted with nanoscale RuO2. ACS Appl Mater Interfaces, 2017, 9: 2387–2395
Cui X, Ren P, Ma C, et al. Robust interface Ru centers for high-per-ormance acidic oxygen evolution. Adv Mater, 2020, 32: 1908126
Audichon T, Guenot B, Baranton S, et al. Effect of the annealing atmosphere on the electrochemical properties of RuO2 nano-oxides synthesized by the instant method. Appl Catal B-Environ, 2017, 218: 385–397
Su J, Ge R, Jiang K, et al. Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: Highly robust electrocatalysts for oxygen evolution in acidic media. Adv Mater, 2018, 30: 1801351
Tian Y, Wang S, Velasco E, et al. A Co-doped nanorod-like RuO2 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. iScience, 2020, 23: 100756
Chen S, Huang H, Jiang P, et al. Mn-doped RuO2 nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media. ACS Catal, 2019, 10: 1152–1160
Retuerto M, Pascual L, Calle-Vallejo F, et al. Na-doped ruthenium perovskite electrocatalysts with improved oxygen evolution activity and durability in acidic media. Nat Commun, 2019, 10: 2041
Wang J, Ji Y, Yin R, et al. Transition metal-doped ultrathin RuO2 networked nanowires for efficient overall water splitting across a broad pH range. J Mater Chem A, 2019, 7: 6411–6416
González-Huerta RG, Ramos-Sánchez G, Balbuena PB. Oxygen evolution in Co-doped RuO2 and IrO2: Experimental and theoretical insights to diminish electrolysis overpotential. J Power Sources, 2014, 268: 69–76
Jiang K, Luo M, Peng M, et al. Dynamic active-site generation of atomic iridium stabilized on nanoporous metal phosphides for water oxidation. Nat Commun, 2020, 11: 2701
Wu G, Chen W, Zheng X, et al. Hierarchical Fe-doped NiOx nanotubes assembled from ultrathin nanosheets containing trivalent nickel for oxygen evolution reaction. Nano Energy, 2017, 38: 167–174
Ge R, Li L, Su J, et al. Ultrafine defective RuO2 electrocatayst integrated on carbon cloth for robust water oxidation in acidic media. Adv Energy Mater, 2019, 9: 1901313
Cao L, Luo Q, Chen J, et al. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat Commun, 2019, 10: 4849
Li G, Li S, Ge J, et al. Discontinuously covered IrO2-RuO2@Ru electrocatalysts for the oxygen evolution reaction: How high activity and long-term durability can be simultaneously realized in the synergistic and hybrid nano-structure. J Mater Chem A, 2017, 5: 17221–17229
Zaman WQ, Wang Z, Sun W, et al. Ni-Co codoping breaks the limitation of single-metal-doped IrO2 with higher oxygen evolution reaction performance and less iridium. ACS Energy Lett, 2017, 2: 2786–2793
Zhu J, Chen Z, Xie M, et al. Iridium-based cubic nanocages with 1.1-nm-thick walls: A highly efficient and durable electrocatalyst for water oxidation in an acidic medium. Angew Chem Int Ed, 2019, 58: 7244–7248
Jiang K, Luo M, Liu Z, et al. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat Commun, 2021, 12: 1687
Yu Y, Jiang K, Luo M, et al. Self-activated catalytic sites on nanoporous dilute alloy for high-efficiency electrochemical hydrogen evolution. ACS Nano, 2021, 15: 5333–5340
Nong HN, Reier T, Oh HS, et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiOx core-shell electrocatalysts. Nat Catal, 2018, 1: 841–851
Li P, Wang M, Duan X, et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat Commun, 2019, 10: 1711
Huang ZF, Song J, Du Y, et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat Energy, 2019, 4: 329–338
Huang ZF, Song J, Dou S, et al. Strategies to break the scaling relation toward enhanced oxygen electrocatalysis. Matter, 2019, 1: 1494–1518
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (51771072), the Outstanding Youth Scientist Foundation of Hunan Province (2020JJ2006), the Fundamental Research Funds for the Central Universities, and the State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body Independent Research Project (71860007). The authors also thank Dr. Ying-Rui Lu and Prof. Ting-Shan Chan for the XAS measurement at Taiwan Light Source.
Author information
Authors and Affiliations
Contributions
Tan Y conceived and supervised this study; Wu Q, Jiang K, Chen D and Lan J carried out the fabrication and characterizations of materials, and electrochemical measurements; Han J conducted the TEM characterizations; Jiang K and Peng M contributed to the XAS measurements and analyses; Tan Y, Jiang K, and Wu Q wrote the paper. All authors contributed to discussions and manuscript review.
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Experimental details and supporting data are available in the online version of the paper.
Qiuli Wu received her MSc degree from Hunan University under the supervisor of Prof. Tan in 2020. Her research interests mainly focus on the preparation and application of nanoporous materials.
Kang Jiang is currently a PhD student under the supervisor of Prof. Tan at the College of Materials Science and Engineering, Hunan University. He received his BE degree from Hunan University in 2018. His current research focuses on the design of 3D nanoporous materials and their applications in electrocatalytic water splitting.
Yongwen Tan is a full professor at the College of Materials Science and Engineering, Hunan University. He received his PhD degree from the College of Materials Science and Engineering, Shanghai Jiao Tong University, China, in 2013. Then, he joined the Advanced Institute for Materials Research (AIMR), Tohoku University as a research associate. His research focuses on the synthesis and applications of 3D nanoporous materials.
Rights and permissions
About this article
Cite this article
Wu, Q., Jiang, K., Han, J. et al. Dynamic shrinkage of metal-oxygen bonds in atomic Co-doped nanoporous RuO2 for acidic oxygen evolution. Sci. China Mater. 65, 1262–1268 (2022). https://doi.org/10.1007/s40843-021-1912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1912-8