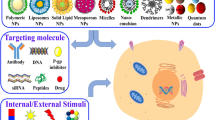
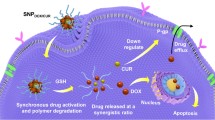
Abstract
Combination chemotherapy is widely exploited to overcome multidrug resistance (MDR) and enhance the therapeutic effect of anti-tumor agents clinically. The traditional combination regimens applied in clinical practice still suffer from various obstacles, such as inevitable side effects. Fortunately, the application of nanotechnology and the proposal of co-delivery systems make the combination therapy more effective. The occurrence, development, and metastasis of tumors are closely related to the cell cycle. The sensitivity of tumor cells to chemotherapeutic drugs can be improved with the cooperation of cell cycle regulators. In this review, the influence of the cell cycle on tumorigenesis and development is introduced briefly. The current strategies of combining chemotherapeutic drugs and cell cycle regulators through co-delivery systems are discussed in detail. We also sketch the possibility of treating tumors mildly via artificially controlling the cell cycle and outline the challenges and perspectives about the improvement of co-delivery systems for cancer therapy.

摘要
联合化疗是临床上用于克服肿瘤多药耐药性、 提高肿瘤治疗效果的常用策略. 然而在临床上, 传统的联合用药仍存在诸多缺陷, 如不可避免的副作用. 纳米技术的应用和多药共同递送体系的提出使联合治疗的治疗效果得以显著提升. 肿瘤的发生、 发展和转移与细胞周期密切相关. 因此, 在化疗过程中配合使用细胞周期调节剂可以增强肿瘤细胞对化疗药物的敏感性. 本综述首先简要介绍了细胞周期对肿瘤发生和发展的影响, 然后详细讨论了目前通过多药共同递送体系结合化疗药物和细胞周期调节剂的一系列策略. 最后, 我们总结概述了通过调控细胞周期进行肿瘤治疗的挑战和前景.
Similar content being viewed by others
References
Randrian V, Biau J, Benoît C, et al. Radiothérapie avec modulation d’intensité préopératoire des cancers rectaux: Intérêt et application. Cancer/Radiothérapie, 2020, 24: 345–353
Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Targeting, 2016, 24: 179–191
Liu YL, Chen D, Shang P, et al. A review of magnet systems for targeted drug delivery. J Control Release, 2019, 302: 90–104
Abdelaziz HM, Gaber M, Abd-Elwakil MM, et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release, 2018, 269: 374–392
Qin T, Xu X, Zhang Z, et al. Paclitaxel/sunitinib-loaded micelles promote an antitumor response in vitro through synergistic immunogenic cell death for triple-negative breast cancer. Nanotechnology, 2020, 31: 365101
Levit SL, Yang H, Tang C. Rapid self-assembly of polymer nanoparticles for synergistic codelivery of paclitaxel and lapatinib via flash nanoprecipitation. Nanomaterials, 2020, 10: 561
Xiong Y, Zhao Y, Miao L, et al. Co-delivery of polymeric metformin and cisplatin by self-assembled core-membrane nanoparticles to treat non-small cell lung cancer. J Control Release, 2016, 244: 63–73
Rozengurt E. Autocrine loops, signal transduction, and cell cycle abnormalities in the molecular biology of lung cancer. Curr Opin Oncology, 1999, 11: 116–122
Ocio EM, Richardson PG, Rajkumar SV, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: A report from the international myeloma working group (IMWG). Leukemia, 2014, 28: 525–542
Zhang X, Xia Q, Wei R, et al. Melatonin protects spermatogonia from the stress of chemotherapy and oxidation via eliminating reactive oxidative species. Free Radical Biol Med, 2019, 137: 74–86
Ferraro G, Loreto D, Merlino A. Interaction of platinum-based drugs with proteins: An overview of representative crystallographic studies. Curr Topics Med Chem, 2021, 21: 6–27
Zhou SF, Wang LL, Di YM, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem, 2008, 15: 1981–2039
Yang L, Wang B, Qiao W, et al. A novel combination chemotherapy integrating with intratumoral chemotherapy. Med Hypotheses, 2009, 73: 334–335
Sarraf CE, Ansari TW, Conway P, et al. Bromodeoxyuridine-labelled apoptosis after treatment with antimetabolites in two murine tumours and in small intestinal crypts. Br J Cancer, 1993, 68: 678–680
Wang X, Tanaka M, Krstin S, et al. The interference of selected cytotoxic alkaloids with the cytoskeleton: An insight into their modes of action. Molecules, 2016, 21: 906
Meng QY, Cong HL, Hu H, et al. Rational design and latest advances of codelivery systems for cancer therapy. Mater Today Bio, 2020, 7: 100056
Xin ZH, Meng YL, Jiang WJ, et al. Finding an efficient tetramethylated hydroxydiethylene of resveratrol analogue for potential anticancer agent. BMC Chem, 2020, 14: 13
Yu X, Li S. Non-metabolic functions of glycolytic enzymes in tumorigenesis. Oncogene, 2017, 36: 2629–2636
Kunnumakkara AB, Bordoloi D, Harsha C, et al. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci, 2017, 131: 1781–1799
Concato VM, Tomiotto-Pellissier F, Silva TF, et al. 3,3′,5,5′-tetramethoxybiphenyl-4,4′-diol induces cell cycle arrest in G2/M phase and apoptosis in human non-small cell lung cancer A549 cells. Chemico-Biol Interact, 2020, 326: 109133
Zhang F, Zhang YY, Sun YS, et al. Asparanin A from Asparagus officinalis L. induces G0/G1 cell cycle arrest and apoptosis in human endometrial carcinoma Ishikawa cells via mitochondrial and PI3K/AKT signaling pathways. J Agric Food Chem, 2020, 68: 213–224
Vessella RL, Pantel K, Mohla S. Tumor cell dormancy: An NCI workshop report. Cancer Biol Ther, 2007, 6: 1492–1500
Zhou Y, Liu Q, Dai X, et al. Transdifferentiation of type II alveolar epithelial cells induces reactivation of dormant tumor cells by enhancing TGF-β1/SNAI2 signaling. Oncol Rep, 2018, 39: 1874–1882
Nie J, Liu L, Zheng W, et al. MicroRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting cyclin D1 and Bcl-2. Carcinogenesis, 2012, 33: 220–225
Xia X, Yu Y, Zhang L, et al. Inhibitor of DNA binding 1 regulates cell cycle progression of endothelial progenitor cells through induction of Wnt2 expression. Mol Med Rep, 2016, 14: 2016–2024
Zhu D, Yuan Y, Qiao J, et al. Enhanced anticancer activity of a protein phosphatase 2A inhibitor on chemotherapy and radiation in head and neck squamous cell carcinoma. Cancer Lett, 2015, 356: 773–780
de Jong Y, Bennani F, van Oosterwijk JG, et al. A screening-based approach identifies cell cycle regulators AURKA, CHK1 and PLK1 as targetable regulators of chondrosarcoma cell survival. J Bone Oncol, 2019, 19: 100268
Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, et al. Targeting cell cycle regulation in cancer therapy. Pharmacol Therapeut, 2013, 138: 255–271
Lim S, Kaldis P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development, 2013, 140: 3079–3093
Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med, 2016, 94: 1313–1326
Murray AW. Recycling the cell cycle. Cell, 2004, 116: 221–234
Elledge SJ. Cell cycle checkpoints: Preventing an identity crisis. Science, 1996, 274: 1664–1672
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem, 2004, 73: 39–85
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med, 2006, 12: 440–450
Khan H, Reale M, Ullah H, et al. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotech Adv, 2020, 38: 107385
Guille A, Chaffanet M, Birnbaum D. Signaling pathway switch in breast cancer. Cancer Cell Int, 2013, 13: 66
Wu Y, Ma J, Sun Y, et al. Effect and mechanism of PI3K/AKT/mTOR signaling pathway in the apoptosis of GC-1 cells induced by nickel nanoparticles. Chemosphere, 2020, 255: 126913
Yu Q, Zeng KW, Ma XL, et al. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int Immunopharmacol, 2016, 38: 104–114
O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu Rev Med, 2015, 66: 311–328
Huang L, Shan YJ, He CX, et al. Effects of L. paracasei subp. paracasei X12 on cell cycle of colon cancer HT-29 cells and regulation of mTOR signalling pathway. J Funct Foods, 2016, 21: 431–439
Elliott B, Millena AC, Matyunina L, et al. Essential role of jund in cell proliferation is mediated via Myc signaling in prostate cancer cells. Cancer Lett, 2019, 448: 155–167
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Seminars Cell Dev Biol, 2018, 80: 50–64
Felty Q, Singh KP, Roy D. Estrogen-induced G1/S transition of G0-arrested estrogen-dependent breast cancer cells is regulated by mitochondrial oxidant signaling. Oncogene, 2005, 24: 4883–4893
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radical Res, 2010, 44: 479–496
Marsh JC. The effects of cancer chemotherapeutic agents on normal hematopoietic precursor cells: A review. Cancer Res, 1976, 36: 1853–1882
Cao R, Peng W, Wang Z, et al. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr Med Chem, 2007, 14: 479–500
Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer, 2012, 12: 104–120
Khoury A, Deo KM, Aldrich-Wright JR. Recent advances in platinum-based chemotherapeutics that exhibit inhibitory and targeted mechanisms of action. J Inorg Biochem, 2020, 207: 111070
Ataei S, Yilmaz S, Ertan-Bolelli T, et al. Generated 3D-common feature hypotheses using the hiphop method for developing new topoisomerase I inhibitors. Arch Pharm Chem Life Sci, 2015, 348: 498–507
Coussy F, El-Botty R, Château-Joubert S, et al. BrCAness, SLFN11, and RB1 loss predict response to topoisomerase I inhibitors in triple-negative breast cancers. Sci Transl Med, 2020, 12: eaax2625
Kim GM, Kim YS, Ae Kang Y, et al. Efficacy and toxicity of belotecan for relapsed or refractory small cell lung cancer patients. J Thorac Oncol, 2012, 7: 731–736
Pommier Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat Rev Cancer, 2006, 6: 789–802
Ma P, Xiao H, Yu C, et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett, 2017, 17: 928–937
Noh J, Kwon B, Han E, et al. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun, 2015, 6: 6907
Costi MP, Tondi D, Rinaldi M, et al. Structure-based studies on species-specific inhibition of thymidylate synthase. Biochim Biophys Acta (BBA)-Mol Basis Dis, 2002, 1587: 206–214
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer, 2003, 3: 330–338
Adjei AA. Pemetrexed (ALIMTA), a novel multitargeted anti-neoplastic agent. Clin Cancer Res, 2004, 10: 4276s-4280s
Abali EE, Skacel NE, Celikkaya H, et al. Regulation of human dihydrofolate reductase activity and expression. Vitam Horm, 2008, 79: 267
Raimondi MV, Randazzo O, La Franca M, et al. DHFR inhibitors: Reading the past for discovering novel anticancer agents. Molecules, 2019, 24: 1140
Zhang L, Guo J, Jiang XM, et al. Identification of nagilactone E as a protein synthesis inhibitor with anticancer activity. Acta Pharmacol Sin, 2020, 41: 698–705
Ferreira R, Schneekloth Jr. JS, Panov KI, et al. Targeting the RNA polymerase I transcription for cancer therapy comes of age. Cells, 2020, 9: 266
Chand S, Mahajan RV, Prasad JP, et al. A comprehensive review on microbial L-asparaginase: Bioprocessing, characterization, and industrial applications. Biotech Appl Biochem, 2020, 67: 619–647
Battogtokh G, Choi YS, Kang DS, et al. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm Sin B, 2018, 8: 862–880
Mordente A, Meucci E, Silvestrini A, et al. Anthracyclines and mitochondria. Adv Exp Med Biol, 2012, 942: 385–419
Gilles A, Frechin L, Natchiar K, et al. Targeting the human 80s ribosome in cancer: From structure to function and drug design for innovative adjuvant therapeutic strategies. Cells, 2020, 9: 629
Burger K, Mühl B, Harasim T, et al. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem, 2010, 285: 12416–12425
Al-Wadei HAN, Al-Wadei MH, Ullah MF, et al. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. PLoS ONE, 2012, 7: e43376
Hsu AL, Ching TT, Wang DS, et al. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem, 2000, 275: 11397–11403
Fukunaga T, Nagahama M, Hatsuzawa K, et al. Implication of sphingolipid metabolism in the stability of the golgi apparatus. J Cell Sci, 2000, 113: 3299–3307
Crespo I, San-Miguel B, Prause C, et al. Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS ONE, 2012, 7: e50407
Peng C, Zhao Y, Hao Y, et al. Syk expression in non-small-cell lung cancer and its relation with angiogenesis. J Can Res Ther, 2016, 12: 663–666
Ye W. The complexity of translating anti-angiogenesis therapy from basic science to the clinic. Dev Cell, 2016, 37: 114–125
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature, 2005, 438: 967–974
Yang JI, Jin B, Kim SY, et al. Antitumour effects of liporaxel (oral paclitaxel) for canine melanoma in a mouse xenograft model. Vet Comp Oncol, 2020, 18: 152–160
Gao P, Wang LL, Liu J, et al. Dihydroartemisinin inhibits endothelial cell tube formation by suppression of the STAT3 signaling pathway. Life Sci, 2020, 242: 117221
Van der Veldt AAM, Lubberink M, Bahce I, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell, 2012, 21: 82–91
Yang WH, Xu J, Mu JB, et al. Revision of the concept of anti-angiogenesis and its applications in tumor treatment. Chronic Dis Transl Med, 2017, 3: 33–40
Freund E, Liedtke KR, Miebach L, et al. Identification of two kinase inhibitors with synergistic toxicity with low-dose hydrogen peroxide in colorectal cancer cells in vitro. Cancers, 2020, 12: 122
Sun M, He L, Fan Z, et al. Effective treatment of drug-resistant lung cancer via a nanogel capable of reactivating cisplatin and enhancing early apoptosis. Biomaterials, 2020, 257: 120252
Deneka AY, Einarson MB, Bennett J, et al. Synthetic lethal targeting of mitotic checkpoints in HPV-negative head and neck cancer. Cancers, 2020, 12: 306
Cong Y, Xiao H, Xiong H, et al. Dual drug backboned shattering polymeric theranostic nanomedicine for synergistic eradication of patient-derived lung cancer. Adv Mater, 2018, 30: 1706220
Moghaddam SV, Abedi F, Alizadeh E, et al. Lysine-embedded cellulose-based nanosystem for efficient dual-delivery of chemotherapeutics in combination cancer therapy. Carbohydr Polym, 2020, 250: 116861
Zhang M, Hagan Iv CT, Min Y, et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials, 2018, 169: 1–10
Rui M, Xin Y, Li R, et al. Targeted biomimetic nanoparticles for synergistic combination chemotherapy of paclitaxel and doxorubicin. Mol Pharm, 2017, 14: 107–123
Li X, Diao W, Xue H, et al. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett, 2020, 489: 163–173
Alle M, G BR, Kim TH, et al. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: Microwave synthesis, characterization, and anti-cancer activity. Carbohydr Polym, 2020, 229: 115511
Gothwal A, Khan I, Gupta U. Polymeric micelles: Recent advancements in the delivery of anticancer drugs. Pharm Res, 2016, 33: 18–39
Li I, Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer, 2019, 18: 32
Liang Y, Zhao X, Ma PX, et al. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci, 2019, 536: 224–234
Nosrati H, Adinehvand R, Manjili HK, et al. Synthesis, characterization, and kinetic release study of methotrexate loaded mPEG-PCL polymersomes for inhibition of MCF-7 breast cancer cell line. Pharm Dev Tech, 2019, 24: 89–98
Qian Q, Zhu L, Zhu X, et al. Drug-polymer hybrid macro-molecular engineering: Degradable PEG integrated by platinum (IV) for cancer therapy. Matter, 2019, 1: 1618–1630
Meng J, Agrahari V, Youm I. Advances in targeted drug delivery approaches for the central nervous system tumors: The inspiration of nanobiotechnology. J Neuroimmune Pharmacol, 2017, 12: 84–98
Fumoto S, Nishida K. Co-delivery systems of multiple drugs using nanotechnology for future cancer therapy. Chem Pharm Bull, 2020, 68: 603–612
Kommineni N, Mahira S, Domb AJ, et al. Cabazitaxel-loaded nanocarriers for cancer therapy with reduced side effects. Pharmaceutics, 2019, 11: 141
Soe ZC, Kwon JB, Thapa RK, et al. Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics, 2019, 11: 63
Wang Y, Ding Y, Xu Y, et al. Mixed micelles of TPGS and Soluplus® for co-delivery of paclitaxel and fenretinide: In vitro and in vivo anticancer study. Pharm Dev Tech, 2020, 25: 865–873
Rawal S, Patel MM. Threatening cancer with nanoparticle aided combination oncotherapy. J Control Release, 2019, 301: 76–109
Qiao Y, Huang X, Nimmagadda S, et al. A robust approach to enhance tumor-selective accumulation of nanoparticles. Oncotarget, 2011, 2: 59–68
Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliver Rev, 2013, 65: 36–48
Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J, 2012, 14: 303–315
Zeng F, Ju RJ, Liu L, et al. Efficacy in treating lung metastasis of invasive breast cancer with functional vincristine plus dasatinib liposomes. Pharmacology, 2018, 101: 43–53
Li C, Han X. Melanoma cancer immunotherapy using PD-L1 siRNA and imatinib promotes cancer-immunity cycle. Pharm Res, 2020, 37: 109
Kumar S, Sharma AR, Sharma G, et al. PLK-1: Angel or devil for cell cycle progression. Biochim Biophys Acta (BBA)-Rev Cancer, 2016, 1865: 190–203
Sizek H, Hamel A, Deritei D, et al. Boolean model of growth signaling, cell cycle and apoptosis predicts the molecular mechanism of aberrant cell cycle progression driven by hyperactive PI3K. PLoS Comput Biol, 2019, 15: e1006402
Bulbake U, Kommineni N, Bryszewska M, et al. Cationic liposomes for co-delivery of paclitaxel and anti-PLK1 siRNA to achieve enhanced efficacy in breast cancer. J Drug Deliver Sci Tech, 2018, 48: 253–265
Li RJ, Ying X, Zhang Y, et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J Control Release, 2011, 149: 281–291
Mohan A, Narayanan S, Balasubramanian G, et al. Dual drug loaded nanoliposomal chemotherapy: A promising strategy for treatment of head and neck squamous cell carcinoma. Eur J Pharm Biopharm, 2016, 99: 73–83
Soe ZC, Thapa RK, Ou W, et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surfs B-Biointerfaces, 2018, 170: 718–728
Ou H, Li J, Chen C, et al. Organic/polymer photothermal nanoagents for photoacoustic imaging and photothermal therapy in vivo. Sci China Mater, 2019, 62: 1740–1758
Aghebati-Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol, 2020, 235: 1962–1972
Sun Q, Zhou Z, Qiu N, et al. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv Mater, 2017, 29: 1606628
Qin SY, Zhang AQ, Cheng SX, et al. Drug self-delivery systems for cancer therapy. Biomaterials, 2017, 112: 234–247
Chen J, Yang X, Huang L, et al. Development of dual-drug-loaded stealth nanocarriers for targeted and synergistic anti-lung cancer efficacy. Drug Deliver, 2018, 25: 1932–1942
Rezvantalab S, Drude NI, Moraveji MK, et al. PLGA-based nanoparticles in cancer treatment. Front Pharmacol, 2018, 9: 1260
Khan I, Joshi G, Nakhate KT, et al. Nano-co-delivery of berberine and anticancer drug using PLGA nanoparticles: Exploration of better anticancer activity and in vivo kinetics. Pharm Res, 2019, 36: 149
Mohammed AFA, Higashi T, Motoyama K, et al. In vitro and in vivo co-delivery of siRNA and doxorubicin by folate-PEG-appended dendrimer/glucuronylglucosyl-β-cyclodextrin conjugate. AAPS J, 2019, 21: 54
Ji Y, Liu X, Li J, et al. Use of ratiometrically designed nanocarrier targeting CDK4/6 and autophagy pathways for effective pancreatic cancer treatment. Nat Commun, 2020, 11: 4249
Wang S, Liu X, Chen S, et al. Regulation of Ca2+ signaling for drug-resistant breast cancer therapy with mesoporous silica nanocapsule encapsulated doxorubicin/siRNA cocktail. ACS Nano, 2019, 13: 274–283
Chen F, Zhang H, Jiang L, et al. Enhancing the cytotoxic efficacy of combined effect of doxorubicin and cyclosporin encapsulated photoluminescent graphene dotted mesoporous nanoparticles against lung cancer cell-specific drug targeting for the nursing care of cancer patients. J Photochem Photobiol B-Biol, 2019, 198: 111578
Du X, Zhang T, Ma G, et al. Glucose-responsive mesoporous silica nanoparticles to generation of hydrogen peroxide for synergistic cancer starvation and chemistry therapy. Int J Nanomed, 2019, Volume 14: 2233–2251
Cagel M, Tesan FC, Bernabeu E, et al. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur J Pharm Biopharm, 2017, 113: 211–228
Banala VT, Urandur S, Sharma S, et al. Targeted co-delivery of the aldose reductase inhibitor epalrestat and chemotherapeutic doxorubicin via a redox-sensitive prodrug approach promotes synergistic tumor suppression. Biomater Sci, 2019, 7: 2889–2906
Chen Y, Zhang W, Huang Y, et al. Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor. Int J Pharm, 2015, 488: 44–58
Debele TA, Yu LY, Yang CS, et al. pH- and GSH-sensitive hyaluronic acid-MP conjugate micelles for intracellular delivery of doxorubicin to colon cancer cells and cancer stem cells. Biomacromolecules, 2018, 19: 3725–3737
Han NN, Li X, Tao L, et al. Doxorubicin and rhein loaded nanomicelles attenuates multidrug resistance in human ovarian cancer. Biochem Biophys Res Commun, 2018, 498: 178–185
Srisa-Nga K, Mankhetkorn S, Okonogi S, et al. Delivery of super-paramagnetic polymeric micelles loaded with quercetin to hepatocellular carcinoma cells. J Pharm Sci, 2019, 108: 996–1006
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules, 2019, 24: 603
Li Z, Guan J. Thermosensitive hydrogels for drug delivery. Expert Opin Drug Deliver, 2011, 8: 991–1007
Lv Q, He C, Quan F, et al. DOX/IL-2/IFN-γ co-loaded thermosensitive polypeptide hydrogel for efficient melanoma treatment. Bioactive Mater, 2018, 3: 118–128
Karavasili C, Andreadis DA, Katsamenis OL, et al. Synergistic antitumor potency of a self-assembling peptide hydrogel for the local co-delivery of doxorubicin and curcumin in the treatment of head and neck cancer. Mol Pharm, 2019, 16: 2326–2341
Wu X, Wu Y, Ye H, et al. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J Control Release, 2017, 255: 81–93
Wu X, He C, Wu Y, et al. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials, 2016, 75: 148–162
Ma H, He C, Cheng Y, et al. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials, 2014, 35: 8723–8734
Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics, 2019, 9: 1015–1028
Luan X, Sansanaphongpricha K, Myers I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin, 2017, 38: 754–763
Liang G, Zhu Y, Ali DJ, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol, 2020, 18: 10
Sharma AK, Prasher P, Aljabali AA, et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv Transl Res, 2020, 10: 1171–1190
Qin Y, Zhang Z, Huang C, et al. Folate-targeted redox-responsive polymersomes loaded with chemotherapeutic drugs and tariquidar to overcome drug resistance. J Biomed Nanotechnol, 2018, 14: 1705–1718
Aarts M, Linardopoulos S, Turner NC. Tumour selective targeting of cell cycle kinases for cancer treatment. Curr Opin Pharmacol, 2013, 13: 529–535
Huang J, Ji G, Xing L, et al. Neo-endocrinochemotherapy: A novel approach for enhancing chemotherapeutic efficacy in clinic? Med Hypotheses, 2013, 80: 441–446
Huang J, Jin L, Ji G, et al. Implication from thyroid function decreasing during chemotherapy in breast cancer patients: Chemosensitization role of triiodothyronine. BMC Cancer, 2013, 13: 334
Conzemius MG, Graham JC, Haynes JS, et al. Effects of treatment with growth hormone and somatostatin on efficacy of diammine [1,1-cyclobutane dicarboxylato (2-)-0,0’]-(SP-4-2) in athymic rats with osteosarcoma. Am J Vet Res, 2000, 61: 646–650
Zou K, Ju JH, Xie H. Pretreatment with insulin enhances anticancer functions of 5-fluorou-racil in human esophageal and colonic cancer cells. Acta Pharmacol Sin, 2007, 28: 721–730
Ijichi K, Adachi M, Ogawa T, et al. Cell-cycle distribution and thymidilate synthatase (TS) expression correlate with 5-FU resistance in head and neck carcinoma cells. Anticancer Res, 2014, 34: 2907–2911
Reinhardt HC, Aslanian AS, Lees JA, et al. P53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell, 2007, 11: 175–189
Murrow LM, Garimella SV, Jones TL, et al. Identification of WEE1 as a potential molecular target in cancer cells by RNAi screening of the human tyrosine kinome. Breast Cancer Res Treat, 2010, 122: 347–357
Jin J, Fang H, Yang F, et al. Combined inhibition of ATR and WEE1 as a novel therapeutic strategy in triple-negative breast cancer. Neoplasia, 2018, 20: 478–488
Sen T, Della Corte CM, Milutinovic S, et al. Combination treatment of the oral CHK1 inhibitor, SRA737, and low-dose gemcitabine enhances the effect of programmed death ligand 1 blockade by modulating the immune microenvironment in SCLC. J Thorac Oncol, 2019, 14: 2152–2163
Riesterer O, Matsumoto F, Wang L, et al. A novel Chk inhibitor, XL-844, increases human cancer cell radiosensitivity through promotion of mitotic catastrophe. Invest New Drugs, 2011, 29: 514–522
Reithofer MR, Valiahdi SM, Galanski M, et al. Novel endothall-containing platinum(IV) complexes: Synthesis, characterization, and cytotoxic activity. Chem Biodiversity, 2008, 5: 2160–2170
Yu CW, Li KKW, Pang SK, et al. Anticancer activity of a series of platinum complexes integrating demethylcantharidin with isomers of 1,2-diaminocyclohexane. Bioorg Med Chem Lett, 2006, 16: 1686–1691
Wang E, Xiong H, Zhou D, et al. Co-delivery of oxaliplatin and demethylcantharidin via a polymer-drug conjugate. Macromol Biosci, 2014, 14: 588–596
Zhou D, Xiao H, Meng F, et al. A polymer-(tandem drugs) conjugate for enhanced cancer treatment. Adv Healthcare Mater, 2013, 2: 822–827
Fan Z, Luo H, Zhou J, et al. Checkpoint kinase 1 inhibition and etoposide exhibit a strong synergistic anticancer effect on chronic myeloid leukemia cell line K562 by impairing homologous recombination DNA damage repair. Oncol Rep, 2020, 44: 2152–2164
Sanij E, Hannan K, Xuan J, et al. Inhibition of RNA polymerase I transcription activates targeted DNA damage response and enhances the efficacy of PARP inhibitors in high-grade serous ovarian cancer. Clin Cancer Res, 2020, 26: 74–75
Tang Xu, Gou X. Is chemotherapy the only option to treat the residual solid tumor cells at the G0 phase after inducing them into the cell cycle? Negative, 2019, 10: 26–28
Milanovic M, Fan DNY, Belenki D, et al. Senescence-associated reprogramming promotes cancer stemness. Nature, 2018, 553: 96–100
Lee JH, Koung FP, Cho CK, et al. Review of tumor dormancy therapy using traditional oriental herbal medicine. J Pharmacopuncture, 2013, 16: 12–20
Nam J, Son S, Park KS, et al. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater, 2019, 4: 398–414
Dai T, Ye F, Hu P, et al. A strategy for enhanced tumor targeting of photodynamic therapy based on Escherichia coli-driven drug delivery system. Sci China Mater, 2021, 64: 232–240
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51703105, 21675091, and 21874078), Taishan Young Scholar Program of Shandong Province (tsqn20161027), the Natural Science Foundation of Shandong Province (ZR2017BEM012), the Major Science and Technology Innovation Project of Shandong Province (2018CXGC1407), the Key Research and Development Project of Shandong Province (2016GGX102028, 2016GGX102039, and 2017GGX20111), China Postdoctoral Science Foundation (2018M630752), the Postdoctoral Scientific Research Foundation of Qingdao, and the First Class Discipline Project of Shandong Province (22074072).
Author information
Authors and Affiliations
Contributions
Author contributions Sun Y and Hu H wrote the original draft; Jing X, Meng Q and Yu B provided some meaningful suggestions for the draft writing; Hu H and Shen Y reviewed and revised the manuscript; Cong H supervised this study.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Ying Sun received her BA degree in polymer material and engineering from Qingdao University in 2019. She is pursuing her MSc degree in materials science in Prof. Cong’s laboratory. Her research focuses on the combination of cell cycle regulation and chemotherapy.
Hao Hu received his PhD degree from Beijing University of Chemical Technology in 2016 with Prof. Fujian Xu. In 2016, he joined the College of Materials Science and Engineering at Qingdao University as a Lecturer. His research focuses on developing smart gene/drug delivery systems for cancer therapy.
Hailin Cong received his PhD degree from Peking University in 2004 with Prof. Weixiao Cao. After completing a Postdoctoral Fellowship at the University of California, Davis, he joined Qingdao University in 2009 as a Distinguished Professor and Distinguished Young Scientist of Shandong Province. His current research interests lie in the synthesis and application of advanced micro-nano materials. He received The Natural Science Award from the Ministry of Education of China (2007). He has served as Member of Editorial Board of Nanoscience & Nanotechnology since 2008, Vice Chair of Editorial Committee of China International Nanoscience and Technology Symposium since 2009, and Member of Editorial Board of Integrated Ferroelectrics since 2012.
Rights and permissions
About this article
Cite this article
Sun, Y., Hu, H., Jing, X. et al. Co-delivery of chemotherapeutic drugs and cell cycle regulatory agents using nanocarriers for cancer therapy. Sci. China Mater. 64, 1827–1848 (2021). https://doi.org/10.1007/s40843-020-1627-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1627-4