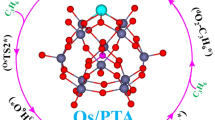
Abstract
Geometric and electronic structures of phosphotungstic acid (PTA) supported single transition metal atom (Fe, Co, Ni, Ru, Rh, Pd, Os, Ir and Pt) catalysts have been systematically investigated by using the first-principles theoretical methods. Possible reaction mechanism for ethylene epoxidation was explored. The most possible anchoring site for the single transition metal atom is the fourfold hollow site on PTA. As the non-noble metal Fe1-PTA system possesses considerable adsorption energies towards both O2 and C2H4, the strong bonding interaction between Fe1 and PTA cluster was analyzed. It is found that the electron transfers from Fe atom to PTA cluster and strong covalent metal-support interactions (CMSI) between the Fe 3d orbitals and O 2p orbitals of PTA lay the foundation of high stability. The proposed catalytic reaction mechanism for ethylene epoxidation on Fe1- PTA single-atom catalyst (SAC) includes three steps: the O2 adsorbs on Fe1-PTA via electron transfer; the first ethylene attacks the adsorbed O2 molecule on Fe1-PTA followed by the formation of C2H4O; finally, the O atom remained on Fe1-PTA reacts with a second ethylene to form the product and accomplish the catalytic cycle. The Fe1-PTA has high selectivity and catalytic activity for ethylene epoxidation via an Eley–Rideal mechanism with low energy barriers. A potentially competitive pathway for the formation of acetaldehyde is not kinetically favorable. These results provide insights for the development of highly efficient heterogeneous SACs for ethylene epoxidation with non-noble metals.
摘要
基于第一性原理, 本文系统研究了过渡金属单原子(铁, 钴, 镍, 钌, 铑, 钯, 锇, 铱, 铂)负载在磷钨酸(PTA)催化剂上的几何和电子结构, 并进一步研究了乙烯环氧化在铁单原子催化剂上的可能反应机理. 我们发现PTA最可能结合过渡金属单原子的位置是四配位中空位. 乙烯环氧化催化活性的理论计算表明, 非贵金属Fe1-PTA具有可观的吸附能, 这是引发此催化循环的关键物理量. 我们进一步进行了铁单原子和PTA结合的成键分析, 发现电荷从铁单原子转移到PTA团簇, 并且强烈的Fe–O共价金属-载体相互作用(CMSI)是其高稳定性的基础. 在催化剂Fe1-PTA上可能的乙烯环氧化催化机理共包括三步: 1) 氧气分子通过电荷转移吸附在Fe1-PTA 上; 2) 第一个乙烯分子攻击吸附在Fe1-PTA上的氧气分子, 随后形成C2H4O; 3) 表面吸附的氧原子和第二个乙烯分子生成C2H4O, 完成催化循环. 本研究发现, Fe1-PTA对于乙烯环氧化主要通过Eley-Rideal 机理进行. 本工作为发展高效多相非贵金属单原子乙烯环氧化催化剂提供了理论依据.
Similar content being viewed by others
References
Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011, 3: 634–641
Yang XF, Wang A, Qiao B, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc Chem Res, 2013, 46: 1740–1748
Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat Rev Chem, 2018, 2: 65–81
Liu J. Catalysis by supported single metal atoms. ACS Catal, 2017, 7: 34–59
Liu JC, Tang Y, Wang YG, et al. Theoretical understanding of the stability of single-atom catalysts. Natl Sci Rev, 2018, 5: 638–641
Liu JC, Wang YG, Li J. Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria. J Am Chem Soc, 2017, 139: 6190–6199
Giannakakis G, Flytzani-Stephanopoulos M, Sykes ECH. Singleatom alloys as a reductionist approach to the rational design of heterogeneous catalysts. Acc Chem Res, 2019, 52: 237–247
Hulsey MJ, Zhang J, Yan N. Harnessing the wisdom in colloidal chemistry to make stable single-atom catalysts. Adv Mater, 2018, 30: 1802304
Qin R, Liu P, Fu G, et al. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods, 2018, 2: 1700286
Qiao B, Liang JX, Wang A, et al. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res, 2015, 8: 2913–2924
Lin J, Wang A, Qiao B, et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J Am Chem Soc, 2013, 135: 15314–15317
Long B, Tang Y, Li J. New mechanistic pathways for CO oxidation catalyzed by single-atom catalysts: supported and doped Au1/ThO2. Nano Res, 2016, 9: 3868–3880
Liang JX, Yang XF, Wang A, et al. Theoretical investigations of non-noble metal single-atom catalysis: Ni1/FeOx for CO oxidation. Catal Sci Technol, 2016, 6: 6886–6892
Deng T, Zheng W, Zhang W. Increasing the range of non-noblemetal single-atom catalysts. Chin J Catal, 2017, 38: 1489–1497
Liu W, Zhang H, Li C, et al. Non-noble metal single-atom catalysts prepared by wet chemical method and their applications in electrochemical water splitting. J Energy Chem, 2020, 47: 333–345
Gebbink R J K, Moret M E. Non-noble Metal Catalysis: Molecular Approaches and Reactions. Weinheim: John Wiley & Sons, 2019
Lv CQ, Liu JH, Guo Y, et al. DFT + U investigation on the adsorption and initial decomposition of methylamine by a Pt singleatom catalyst supported on rutile (110) TiO2. Appl Surf Sci, 2016, 389: 411–418
Song Z, Li W, Niu F, et al. A novel method to decorate Au clusters onto graphene via a mild Co-reduction process for ultrahigh catalytic activity. J Mater Chem A, 2017, 5: 230–239
Kwak JH, Lee J, Szanyi J, et al. Modification of the acid/base properties of γ-Al2O3 by oxide additives: An ethanol TPD investigation. Catal Today, 2016, 265: 240–244
Peterson EJ, De LaRiva AT, Lin S, et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat Commun, 2014, 5: 4885
Li J, Liu J, Zhang T. Preface to the special issue of the international symposium on single-atom catalysis (ISSAC-2016). Chin J Catal, 2017, 38: 1431
Liang JX, Wang YG, Yang XF, et al. Recent advances in singleatom catalysis. Encyclopedia Inorg Bioinorg Chem, 2011, 1–11
Zheng N, Zhang T. Preface: single-atom catalysts as a new generation of heterogeneous catalysts. Natl Sci Rev, 2018, 5: 625
Liu L, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem Rev, 2018, 118: 4981–5079
Chen Y, Ji S, Chen C, et al. Single-atom catalysts: synthetic strategies and electrochemical applications. Joule, 2018, 2: 1242–1264
Jones TE, Wyrwich R, Bocklein S, et al. The selective species in ethylene epoxidation on silver. ACS Catal, 2018, 8: 3844–3852
Ozbek MO, Onal I, van Santen RA. Ethylene epoxidation catalyzed by silver oxide. ChemCatChem, 2011, 3: 150–153
Christopher P, Linic S. Engineering selectivity in heterogeneous catalysis: Ag nanowires as selective ethylene epoxidation catalysts. J Am Chem Soc, 2008, 130: 11264–11265
Linic S, Barteau MA. Control of ethylene epoxidation selectivity by surface oxametallacycles. J Am Chem Soc, 2003, 125: 4034–4035
van Hoof AJF, Filot IAW, Friedrich H, et al. Reversible restructuring of silver particles during ethylene epoxidation. ACS Catal, 2018, 8: 11794–11800
Lopez X, Carbo JJ, Bo C, et al. Structure, properties and reactivity of polyoxometalates: A theoretical perspective. Chem Soc Rev, 2012, 41: 7537
Hossain MM, Aldous L. Polyoxometalates as solution-phase electrocatalytic mediators for reduced electrode fouling and the improved oxidative response of phenols. Electrochem Commun, 2016, 69: 32–35
Mei Y, Huang W, Yang Z, et al. Ion-pairing and aggregation of ionic liquid-neutralized polyoxometalate salts in aqueous solutions. Fluid Phase Equilib, 2016, 425: 31–39
Wen S, Guan W, Wang J, et al. Theoretical investigation of structural and electronic propertyies of [PW12O40]3− on graphene layer. Dalton Trans, 2012, 41: 4602–4607
Xie L, Jiang R, Zhu F, et al. Application of functionalized magnetic nanoparticles in sample preparation. Anal Bioanal Chem, 2014, 406: 377–399
Hill CL. Introduction: Polyoxometalates multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem Rev, 1998, 98: 1–2
Dolbecq A, Dumas E, Mayer CR, et al. Hybrid organic−inorganic polyoxometalate compounds: from structural diversity to applications. Chem Rev, 2010, 110: 6009–6048
Zhang Z, Wang YL, Yang GY. Two inorganic-organic hybrid polyoxotungstates constructed from tetra-ZrIV-substituted sandwich- type germanotungstates functionalized by tris ligand. InOrg Chem Commun, 2017, 85: 32–36
Coperet C, Comas-Vives A, Conley MP, et al. Surface organometallic and coordination chemistry toward single-site heterogeneous catalysts: strategies, methods, structures, and activities. Chem Rev, 2016, 116: 323–421
Laird T. Dangers of the unknown. Org Process Res Dev, 2003, 7: 225
Tanielyan SK, Augustine RL, Marin N, et al. Anchored Wilkinson catalyst. ACS Catal, 2011, 1: 159–169
Song CE, Lee SG. Supported chiral catalysts on inorganic materials. Chem Rev, 2002, 102: 3495–3524
Wu Y, Wang D, Li Y. Understanding of the major reactions in solution synthesis of functional nanomaterials. Sci China Mater, 2016, 59: 938–996
Allen DT. Luque, Yan, You: 2018 winners of the ACS Sustainable Chemistry & Engineering lectureship awards. ACS Sustain Chem Eng, 2017, 5: 7450
Roithova J, Schroder D. Selective activation of alkanes by gas-phase metal ions. Chem Rev, 2010, 110: 1170–1211
Mallat T, Baiker A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem Rev, 2004, 104: 3037–3058
Zhang Z, Qu Y, Wang S, et al. Theoretical study on the mechanisms of the conversion of methyl lactate over sodium polyphosphate catalyst. J Mol Catal A-Chem, 2010, 323: 91–100
Yang X, Waters T, Wang XB, et al. Photoelectron spectroscopy of free polyoxoanions M6O192− and W6O192− in the gas phase. J Phys Chem A, 2004, 108: 10089–10093
Li J. Electronic structures, (d-p) π conjugation effects, and spectroscopic properties of polyoxometalates: M6O192− (M = Cr, Mo, W). J Cluster Sci, 2002, 13: 137–163
Lopez X, Maestre JM, Bo C, et al. Electronic properties of polyoxometalates: A DFT study of α/β-[XM12O40]n− relative stability (M = W, Mo and X a main group element). J Am Chem Soc, 2001, 123: 9571–9576
He P, Xu B, Xu X, et al. Surfactant encapsulated palladium-polyoxometalates: controlled assembly and their application as singleatom catalysts. Chem Sci, 2016, 7: 1011–1015
Zhang B, Asakura H, Yan N. Atomically dispersed rhodium on self-assembled phosphotungstic acid: structural features and catalytic CO oxidation properties. Ind Eng Chem Res, 2017, 56: 3578–3587
Zhang B, Asakura H, Zhang J, et al. Stabilizing a platinum1 singleatom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew Chem Int Ed, 2016, 55: 8319–8323
Hulsey MJ, Zhang B, Ma Z, et al. In situ spectroscopy-guided engineering of rhodium single-atom catalysts for CO oxidation. Nat Commun, 2019, 10: 1330
Zhang B, Sun G, Ding S, et al. Atomically dispersed Pt1–poly- oxometalate catalysts: How does metal–support interaction affect stability and hydrogenation activity? J Am Chem Soc, 2019, jacs.9b00486
Yu MA, Feng Y, Gao L, et al. Phosphomolybdic acid supported single-metal-atom catalysis in CO oxidation: First-principles calculations. Phys Chem Chem Phys, 2018, 20: 20661–20668
Kozhevnikov IV. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem Rev, 1998, 98: 171–198
Yue ZC, Du HJ, Li L, et al. Synthesis and characterization of two new polymolybdate clusters containing chlorine based on Mo-POMs and N-heterocycle templates. Synthesis Reactivity InOrg Metal-Org Nano-Metal Chem, 2014, 44: 678–686
Tessonnier JP, Goubert-Renaudin S, Alia S, et al. Structure, stability, and electronic interactions of polyoxometalates on functionalized graphene sheets. Langmuir, 2013, 29: 393–402
Matthey J, Aesar A. PGMs in the lab: Platinum group metals in polyoxometalates. Platinum Met Rev, 2014, 58: 40–41
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1997, 77: 3865–3868
Kresse G, Furthmuller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci, 1996, 6: 15–50
Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys Rev B, 1993, 47: 558–561
Kresse G. Ab initio molecular dynamics for liquid metals. J Non- Crystalline Solids, 1995, 192–193: 222–229
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B, 1999, 59: 1758–1775
Blochl PE. Projector augmented-wave method. Phys Rev B, 1994, 50: 17953–17979
Perdew JP, Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B, 1992, 45: 13244–13249
Tang W, Sanville E, Henkelman G. A grid-based Bader analysis algorithm without lattice bias. J Phys-Condens Matter, 2009, 21: 084204
Yu M, Trinkle DR. Accurate and efficient algorithm for Bader charge integration. J Chem Phys, 2011, 134: 064111
Henkelman G, Jonsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys, 2000, 113: 9978–9985
Henkelman G, Uberuaga BP, Jonsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys, 2000, 113: 9901–9904
Henkelman G, Jonsson H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys, 1999, 111: 7010–7022
Kastner J, Sherwood P. Superlinearly converging dimer method for transition state search. J Chem Phys, 2008, 128: 014106
Su J, Hu S, Huang W, et al. On the oxidation states of metal elements in MO3 − (M=V, Nb, Ta, Db, Pr, Gd, Pa) anions. Sci China Chem, 2016, 59: 442–451
Ma XL, Liu JC, Xiao H, et al. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J Am Chem Soc, 2018, 140: 46–49
Liu JC, Ma XL, Li Y, et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat Commun, 2018, 9: 1610
Acknowledgements
This work was financially supported by the Na Acknowledgements This work was financially supported by the National Natural Science Foundation of China (21590792, 91426302 and 21433005) to Li J. The support from Guangdong Provincial Key Laboratory of Catalysis (2020B121201002) is also acknowledged. The calculations were performed using supercomputers at Tsinghua National Laboratory for Information Science and Technology. Yu X thanks for the Special Funding for Transformation of Scientific and Technological Achievements in Qinghai Province (2018-GX-101), and the Natural Science Basic Research Program of Shaanxi Province (2019JM-226).
Author information
Authors and Affiliations
Contributions
Li J designed the research. Talib SH and Yu X performed the calculations. Talib SH, Yu X, Yu Q, Baskaran S, and Li J interpreted the data. Talib SH, Yu X, Yu Q and Li J co-wrote and revised the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Shamraiz Hussain Talib is currently a PhD candidate at the Department of Chemistry and Key Laboratory of Organic Optoelectronics & Molecular Engineering, Tsinghua University, under the supervision of Prof. Jun Li. He received his M. Phil degree (majored in chemistry) from the Department of Chemistry, Mohi-Ud-Din Islamic University, AJ&K, Pakistan in 2016. His PhD research focuses on the theoretical investigations on heterogeneous single-atom catalysts.
Xiaohu Yu received his PhD degree from the Institute of Coal Chemistry, Chinese Academy of Sciences in 2013. He did postdoctoral research at Moscow Institute of Physics and Technology from 2013 to 2015. He worked as a visiting scholar in Prof. Jun Li’s group at Tsinghua University from 2019 to 2020. He is now an associative professor at Shaanxi University of Technology. His research interests focus on theoretical inorganic chemistry and computational cataysis science.
Jun Li received his PhD degree from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences in 1992. He did postdoctoral research at the University of Siegen and Ohio State University from 1994 to 1997. He worked as a research scientist at Ohio State University and senior research scientist at the Pacific Northwest National Laboratory from 1997 to 2009. He is now a full professor at Tsinghua University. His research involves theoretical chemistry, heavy-element chemistry, and computational catalysis science.
Supplementary information
40843_2020_1399_MOESM1_ESM.pdf
Non-noble metal single-atom catalysts with phosphotungstic acid (PTA) support: A theoretical study of ethylene epoxidation
Rights and permissions
About this article
Cite this article
Talib, S.H., Yu, X., Yu, Q. et al. Non-noble metal single-atom catalysts with phosphotungstic acid (PTA) support: A theoretical study of ethylene epoxidation. Sci. China Mater. 63, 1003–1014 (2020). https://doi.org/10.1007/s40843-020-1399-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1399-y