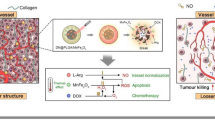
Abstract
The anti-vascular therapy has been extensively studied for high performance tumor therapy by suppressing the tumor angiogenesis or cutting off the existing tumor vasculature. We have previously reported a novel anti-tumor treatment technique using radiofrequency (RF)-assisted gadofullerene nanocrystals (GFNCs) to selectively disrupt the tumor vasculature. In this work, we further revealed the changes on morphology and functionality of the tumor vasculature during the high-performance RF-assisted GFNCs treatment in vivo. Here, a clearly evident mechanism of this technique in tumor vascular disruption was elucidated. Based on the H22 tumor bearing mice with dorsal skin flap chamber (DSFC) model and the dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) technique, it was revealed that the GFNCs would selectively inset in the gaps of tumor vasculature due to the innately incomplete structures and unique microenvironment of tumor vasculature, and they damaged the surrounding endothelia cells excited by the RF to induce a phase transition accompanying with size expansion. Soon afterwards, the blood flow of the tumor blood vessels was permanently shut off, causing the entire tumor vascular network to collapse within 24 h after the treatment. The RF-assistant GFNCs technique was proved to aim at the tumor vasculature precisely, and was harmless to the normal vasculature. The current studies provide a rational explanation on the high efficiency anticancer activity of the RF-assisted GFNCs treatment, suggesting a novel technique with potent clinical application.
摘要
射频辅助金属富勒烯纳米晶体阻断肿瘤血管作为一项新兴的抗肿瘤技术, 因其高效安全的作用效果, 在癌症治疗的研究发展过程中表现出巨大的应用前景. 本文针对该技术, 提出了对其阻断肿瘤血管的实时原位研究方法, 清晰明确地揭示了高效靶向阻断肿瘤血管的机制. 通过建立小鼠肿瘤背部皮翼视窗模型, 实现了在治疗过程中肿瘤血管和正常血管的形态变化及血流情况的直观监测评价. 同时, 采用临床常用的动态增强磁共振成像手段对肿瘤血管功能进行实时定量评估, 借助相关参数Ktrans, 证明了肿瘤血管在治疗后发生了持续不可逆的破坏. 具体表现为局部肿瘤血管出血、 塌陷, 导致整个肿瘤血管网的血流停止, 切断了肿瘤组织与外界的营养交换, 进而致使肿瘤坏死, 而正常血管并不会受到损伤. 此研究结果是对该技术高效靶向治疗肿瘤的深入研究, 有利于促进其在临床上的转化和应用.
Article PDF
Similar content being viewed by others
References
Margulis K, Neofytou EA, Beygui RE, et al. Celecoxib nanoparticles for therapeutic angiogenesis. ACS Nano, 2015, 9: 9416–9426
Nagy JA, Dvorak HF. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis, 2012, 29: 657–662
Voronin DV, Sindeeva OA, Kurochkin MA, et al. In vitro and in vivo visualization and trapping of fluorescent magnetic microcapsules in a bloodstream. ACS Appl Mater Interfaces, 2017, 9: 6885–6893
Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res, 1991, 51: 5054–5059
Dvorak HF, Nagy JA, Dvorak J, et al. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol, 1988, 133: 95–109
Kobayashi H, Tsuruchi N, Sugihara K, et al. Expression of α-smooth muscle actin in benign or malignant ovarian tumors. Gynecologic Oncology, 1993, 48: 308–313
Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer, 2005, 5: 423–435
Tozer G, Lewis S, Michalowski A, et al. The relationship between regional variations in blood flow and histology in a transplanted rat fibrosarcoma. Br J Cancer, 1990, 61: 250–257
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature, 2000, 407: 249–257
Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res, 1990, 50: 4478–4484
Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA, 1998, 95: 4607–4612
Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol, 2000, 156: 1363–1380
Baluk P, Morikawa S, Haskell A, et al. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol, 2003, 163: 1801–1815
Yan X, Yu Q, Guo L, et al. Positively charged combinatory drug delivery systems against multi-drug-resistant breast cancer: beyond the drug combination. ACS Appl Mater Interfaces, 2017, 9: 6804–6815
Kretzschmann VK, Fürst R. Plant-derived vascular disrupting agents: compounds, actions, and clinical trials. Phytochem Rev, 2014, 13: 191–206
Thorpe PE. Vascular targeting agents as cancer therapeutics. Clinical Cancer Res, 2004, 10: 415–427
Pilat MJ, Lorusso PM. Vascular disrupting agents. J Cell Biochem, 2006, 99: 1021–1039
Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov, 2010, 9: 790–803
Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncology, 2003, 4: 141–148
Ching LM, Zwain S, Baguley BC. Relationship between tumour endothelial cell apoptosis and tumour blood flow shutdown following treatment with the antivascular agent DMXAA in mice. Br J Cancer, 2004, 90: 906–910
Cooney MM, van Heeckeren W, Bhakta S, et al. Drug insight: vascular disrupting agents and angiogenesis—novel approaches for drug delivery. Nat Clin Prac Oncol, 2006, 3: 682–692
Zhen M, Shu C, Li J, et al. A highly efficient and tumor vasculartargeting therapeutic technique with size-expansible gadofullerene nanocrystals. Sci China Mater, 2015, 58: 799–810
Li J, Guan M, Wang T, et al. Gd@C82-(ethylenediamine)8 nanoparticle: a new high-efficiency water-soluble ROS scavenger. ACS Appl Mater Interfaces, 2016, 8: 25770–25776
Zhang Y, Shu C, Zhen M, et al. A novel bone marrow targeted gadofullerene agent protect against oxidative injury in chemotherapy. Sci China Mater, 2017, 60: 866–880
Chan LS, Malcontenti-Wilson C, Muralidharan V, et al. Alterations in vascular architecture and permeability following OXi4503 treatment. Anti-Cancer Drugs, 2008, 19: 17–22
El-Emir E, Boxer GM, Petrie IA, et al. Tumour parameters affected by combretastatin A-4 phosphate therapy in a human colorectal xenograft model in nude mice. Eur J Cancer, 2005, 41: 799–806
Jiang W, Huang Y, An Y, et al. Remodeling tumor vasculature to enhance delivery of intermediate-sized nanoparticles. ACS Nano, 2015, 9: 8689–8696
Malamas AS, Jin E, Gujrati M, et al. Dynamic contrast enhanced MRI assessing the antiangiogenic effect of silencing HIF-1α with targeted multifunctional ECO/siRNA nanoparticles. Mol Pharm, 2016, 13: 2497–2506
Shenoi MM, Iltis I, Choi J, et al. Nanoparticle delivered vascular disrupting agents (VDAs): use of TNF-α conjugated gold nanoparticles for multimodal cancer therapy. Mol Pharm, 2013, 10: 1683–1694
Tozer GM, Akerman S, Cross NA, et al. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res, 2008, 68: 2301–2311
Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res, 2006, 12: 4090–4094
Kötz B, West C, Saleem A, et al. Blood flow and Vd (water): both biomarkers required for interpreting the effects of vascular targeting agents on tumor and normal tissue. Mol Cancer Therapeutics, 2009, 8: 303–309
Wu X, Jeong EK, Emerson L, et al. Noninvasive evaluation of antiangiogenic effect in a mouse tumor model by DCE-MRI with Gd-DTPA cystamine copolymers. Mol Pharm, 2010, 7: 41–48
Marzola P, Degrassi A, Calderan L, et al. Early antiangiogenic activity of SU11248 evaluated in vivo by dynamic contrast-enhanced magnetic resonance imaging in an experimental model of colon carcinoma. Clinical Cancer Res, 2005, 11: 5827–5832
Mikawa M, Kato H, Okumura M, et al. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjugate Chem, 2001, 12: 510–514
Leunig M, Yuan F, Menger MD, et al. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res, 1992, 52: 6553–6560
Kohtala S, Theilmann W, Suomi T, et al. Brief isoflurane anesthesia produces prominent phosphoproteomic changes in the adult mouse hippocampus. ACS Chem Neurosci, 2016, 7: 749–756
Daldrup H, Shames DM, Wendland M, et al. Correlation of dynamic contrast-enhanced MR imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media. Am J Roentgenology, 1998, 171: 941–949
Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imag, 1999, 10: 223–232
Kokubo K, Shirakawa S, Kobayashi N, et al. Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res, 2011, 4: 204–215
Zhang J, Ye Y, Chen Y, et al. Gd3N@C84(OH)x: A new egg-shaped metallofullerene magnetic resonance imaging contrast agent. J Am Chem Soc, 2014, 136: 2630–2636
Zahra MA, Hollingsworth KG, Sala E, et al. Dynamic contrastenhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncology, 2007, 8: 63–74
Malamas AS, Jin E, Zhang Q, et al. Anti-angiogenic effects of bumetanide revealed by DCE-MRI with a biodegradable macromolecular contrast agent in a colon cancer model. Pharm Res, 2015, 32: 3029–3043
Pedersen M, Morkenborg J, Jensen FT, et al. In vivo measurements of relaxivities in the rat kidney cortex. J Magn Reson Imag, 2000, 12: 289–296
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51472248 and 51502301), National Major Scientific Instruments and Equipments Development Project (ZDYZ2015-2), and the Key Research Program of the Chinese Academy of Sciences (QYZDJ-SSW-SLH025). We thank Zhentao Zuo for the design of the radiofrequency generator and the help by the State Key Lab of Brain & Cognitive Sciencesin Institute of Biophysics, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ruijun Deng was born in 1991. She received her bachelor degree from Hebei University. She is currently pursuing her PhD degree in physical chemistry from the Institute of Chemistry, Chinese Academy of Sciences. Her main research includes the biomedical application of the fullerenes and gadofullerenes.
Yuqing Wang was born in 1980. He received his PhD degree in biomedical engineering from the University of Electronic Science and Technology in 2011. Currently, he is an engineer at the National Center for Nanoscience and Technology. His research interests refer to medical imaging processing in MR.
Mingming Zhen was born in 1987. She received her PhD degree in physical chemistry from the Institute of Chemistry, Chinese Academy of Sciences in 2014. Currently, she is an assistant professor at the Institute of Chemistry, Chinese Academy of Sciences. Her research interests include biomedical applications of fullerenes and gadofullerenes.
Chunru Wang was born in 1965. He received his PhD degree in physical chemistry from Dalian Institute of Chemistry Physics, Chinese Academy of Sciences in 1992. Currently, he is a professor at the Institute of Chemistry, Chinese Academy of Sciences. His research interests include fullerenes and endohedral fullerenes, mainly focusing on their industrialization and applications. He discovered the metal carbide fullerenes for the first time, researched on high efficiency MRI contrast agents and developed a novel tumor vascular-targeting therapy technique using gadofullerenes.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Deng, R., Wang, Y., Zhen, M. et al. Real-time monitoring of tumor vascular disruption induced by radiofrequency assisted gadofullerene. Sci. China Mater. 61, 1101–1111 (2018). https://doi.org/10.1007/s40843-017-9223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9223-6