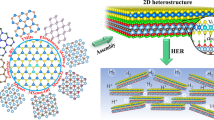
Abstract
The development of ultrasmall transition-metal dichalcogenide (such as MoS2, MoSe2) nanostructures is an efficient strategy to increase the active edge sites and overall performance for hydrogen evolution reaction. Here, we report an in-situ tearing strategy to produce the carbon nanotube supported subnanometer ternary MoSeS (denoted as CNTs@NiSe@MoSeS) for efficient hydrogen evolution. Large (18.3 ± 1.1 nm in length) multilayer MoS2 sheets grown on Ni (OH)2 thin film are torn into subnanometer (5.2 ± 0.7 nm in length) MoSeS via a subsequent selenization progress, along with the transformation of Ni(OH)2 thin film into small NiSe nanoplates. The resulting nanocomposite exhibits abundant active edge sites, outstanding 10,000-cycle stability and ultrahigh activity with a low overpotential of 189 mV at a high current density of 200 mA cm−2 toward hydrogen evolution.
摘要
制备超小型层状金属硫属化合物是一种有效增加边缘活性位点和高效提升析氢性能的方法. 本文首次报道了一种原位撕裂的方法来制备由碳纳米管(CNTs)支撑的三元MoSeS纳米复合析氢催化剂. 生长在CNTs@Ni(OH)2薄膜上的大尺寸多层MoS2片(18.3± 1.1 nm), 通过原位的硒化过程被撕裂成超小的MoSeS(5.2 ± 0.7nm)纳米片, 同时Ni(OH)2薄膜也被转化为小尺寸的NiSe纳米晶, 最终得到的纳米复合催化剂具有丰富的边缘活性位点,表现出超高的活性, 在200 mA cm−2高的电流密度下, 过电势只要189 mV; 同时在10000次循环下, 依旧保持优异的稳定性. 该工作为纳米晶催化剂的设计和应用打下了良好的基础.
Similar content being viewed by others
References
Kong D, Wang H, Lu Z, et al. CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc, 2014, 136: 4897–4900
Faber MS, Dziedzic R, Lukowski MA, et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J Am Chem Soc, 2014, 136: 10053–10061
Wang DY, Gong M, Chou HL, et al. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets–carbon nanotubes for hydrogen evolution reaction. J Am Chem Soc, 2015, 137: 1587–1592
Cheng L, Huang W, Gong Q, et al. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction. Angew Chem Int Ed, 2014, 53: 7860–7863
Deng J, Ren P, Deng D, et al. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew Chem Int Ed, 2015, 54: 2100–2104
Jiang P, Liu Q, Liang Y, et al. A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew Chem Int Ed, 2014, 53: 12855–12859
Popczun EJ, Read CG, Roske CW, et al. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew Chem Int Ed, 2014, 53: 5427–5430
Yang J, Voiry D, Ahn SJ, et al. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew Chem Int Ed, 2013, 52: 13751–13754
Wang P, Jiang K, Wang G, et al. Phase and interface engineering of platinum-nickel nanowires for efficient electrochemical hydrogen evolution. Angew Chem Int Ed, 2016, 55: 12859–12863
Zhang B, Wang HH, Su H, et al. Nitrogen-doped graphene microtubes with opened inner voids: highly efficient metal-free electrocatalysts for alkaline hydrogen evolution reaction. Nano Res, 2016, 9: 2606–2615
Lu S, Zhuang Z. Electrocatalysts for hydrogen oxidation and evolution reactions. Sci China Mater, 2016, 59: 217–238
Zheng Y, Jiao Y, Zhu Y, et al. Hydrogen evolution by a metal-free electrocatalyst. Nat Commun, 2014, 5: 3783
Sheng W, Zhuang Z, Gao M, et al. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat Commun, 2015, 6: 5848–5853
Huang X, Zeng Z, Bao S, et al. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat Commun, 2013, 4: 1444–1451
Zhang Y, Gong Q, Li L, et al. MoSe2 porous microspheres comprising monolayer flakes with high electrocatalytic activity. Nano Res, 2015, 8: 1108–1115
Yang H, Zhang Y, Hu F, et al. Urchin-like CoP nanocrystals as hydrogen evolution reaction and oxygen reduction reaction dualelectrocatalyst with superior stability. Nano Lett, 2015, 15: 7616–7620
Li H, Wang L. NaYF4:Yb3+/Er3+ nanoparticle-based upconversion luminescence resonance energy transfer sensor for mercury(ii) quantification. Analyst, 2013, 138: 1589–1595
Cummins DR, Martinez U, Sherehiy A, et al. Efficient hydrogen evolution in transition metal dichalcogenides via a simple one-step hydrazine reaction. Nat Commun, 2016, 7: 11857–11866
Liu D, Xu W, Liu Q, et al. Unsaturated-sulfur-rich MoS2 nanosheets decorated on free-standing SWNT film: Synthesis, characterization and electrocatalytic application. Nano Res, 2016, 9: 2079–2087
Thoi VS, Sun Y, Long JR, et al. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem Soc Rev, 2013, 42: 2388–2400
Sun Y, Gao S, Lei F, et al. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem Soc Rev, 2015, 44: 623–636
Xie J, Zhang H, Li S, et al. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater, 2013, 25: 5807–5813
Tang YJ, Wang Y, Wang XL, et al. Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution. Adv Energ Mater, 2016, 6: 1600116
Xie J, Qu H, Xin J, et al. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction. Nano Res, 2017, 10: 1178–1188
Yang Y, Xu X, Wang X. Synthesis of Mo-based nanostructures from organic-inorganic hybrid with enhanced electrochemical for water splitting. Sci China Mater, 2015, 58: 775–784
Shima S, Pilak O, Vogt S, et al. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science, 2008, 321: 572–575
Asadi M, Kumar B, Behranginia A, et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat Commun, 2014, 5: 4470–4477
Zheng Y, Jiao Y, Jaroniec M, et al. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew Chem Int Ed, 2015, 54: 52–65
Jaramillo TF, Jørgensen KP, Bonde J, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science, 2007, 317: 100–102
Karunadasa HI, Montalvo E, Sun Y, et al. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science, 2012, 335: 698–702
Hinnemann B, Moses PG, Bonde J, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc, 2005, 127: 5308–5309
Tsai C, Abild-Pedersen F, Nørskov JK. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett, 2014, 14: 1381–1387
Hong X, Liu J, Zheng B, et al. A universal method for preparation of noble metal nanoparticle-decorated transition metal dichalcogenide nanobelts. Adv Mater, 2014, 26: 6250–6254
Zhang X, Lai Z, Liu Z, et al. A facile and universal top-down method for preparation of monodisperse transition-metal dichalcogenide nanodots. Angew Chem Int Ed, 2015, 54: 5425–5428
Gao MR, Chan MKY, Sun Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat Commun, 2015, 6: 7493–7500
Lukowski MA, Daniel AS, Meng F, et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc, 2013, 135: 10274–10277
Voiry D, Salehi M, Silva R, et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett, 2013, 13: 6222–6227
Geng X, Wu W, Li N, et al. Three-dimensional structures of MoS2 nanosheets with ultrahigh hydrogen evolution reaction in water reduction. Adv Funct Mater, 2014, 24: 6123–6129
Wang H, Lu Z, Kong D, et al. Electrochemical tuning of MoS2 nanoparticles on three-dimensional substrate for efficient hydrogen evolution. ACS Nano, 2014, 8: 4940–4947
Liao L, Zhu J, Bian X, et al. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv Funct Mater, 2013, 23: 5326–5333
Zhang C, Li J, Shi C, et al. Effect of Ni, Fe and Fe-Ni alloy catalysts on the synthesis of metal contained carbon nano-onions and studies of their electrochemical hydrogen storage properties. J Energ Chem, 2014, 23: 324–330
Chang YH, Lin CT, Chen TY, et al. Highly efficient electrocatalytic hydrogen production by MoSx grown on graphene-protected 3D Ni foams. Adv Mater, 2013, 25: 756–760
Cui J, Jiang R, Lu W, et al. Plasmon-enhanced photoelectrical hydrogen evolution on monolayer MoS2 decorated Cu1.75S-Au nanocrystals. Small, 2017, 13: 1602235–1602241
Kibsgaard J, Chen Z, Reinecke BN, et al. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat Mater, 2012, 11: 963–969
Seo B, Jung GY, Sa YJ, et al. Monolayer-precision synthesis of molybdenum sulfide nanoparticles and their nanoscale size effects in the hydrogen evolution reaction. ACS Nano, 2015, 9: 3728–3739
Xu J, Cui J, Guo C, et al. Ultrasmall Cu7S4@MoS2 hetero-nanoframes with abundant active edge sites for ultrahigh-performance hydrogen evolution. Angew Chem Int Ed, 2016, 55: 6502–6505
Li DJ, Maiti UN, Lim J, et al. Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett, 2014, 14: 1228–1233
Merki D, Fierro S, Vrubel H, et al. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem Sci, 2011, 2: 1262–1267
Li Y, Wang H, Xie L, et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc, 2011, 133: 7296–7299
Zhang X, Lai Z, Tan C, et al. Solution-processed two-dimensional MoS2 nanosheets: preparation, hybridization, and applications. Angew Chem Int Ed, 2016, 55: 8816–8838
Shi Y, Wang Y, Wong JI, et al. Self-assembly of hierarchical MoSx/CNT nanocomposites (2<x<3): towards high performance anode materials for lithium ion batteries. Sci Rep, 2013, 3: 2169–2176
Li H, Yu K, Li C, et al. Charge-transfer induced high efficient hydrogen evolution of MoS2/graphene cocatalyst. Sci Rep, 2016, 5: 18730
Conway BE, Tilak BV. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim Acta, 2002, 47: 3571–3594
Xie J, Zhang J, Li S, et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J Am Chem Soc, 2013, 135: 17881–17888
Acknowledgements
This research was supported in part by the National Natural Science Foundation of China (21475007 and 21675009), and the Fundamental Research Funds for the Central Universities (buctrc201608 and buctrc201720). We also thank the support from the “Public Hatching Platform for Recruited Talents of Beijing University of Chemical Technology”.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wenli Lu is currently a Master candidate in chemistry under the supervision of Prof. Leyu Wang at Beijing University of Chemical Technology (BUCT) since 2015. His research interest is focused on the fabrication of functional nanostructures for electrocatalysis.
Leyu Wang is a professor of chemistry at BUCT. He received his PhD in chemistry from Tsinghua University with Prof. Yadong Li in 2007. Then he joined Prof. Huang’s group at the University of California at Los Angeles (UCLA) as a postdoctoral researcher from 2007 to 2009. He moved to BUCT’s Chemistry Department in October 2009. His research interests span from the controlled synthesis of upconversion luminescence nanoparticles (UCNPs), localized surface plasmon resonance (LSPR) near-infrared (NIR) semiconductor NPs, magnetic nanomaterials, metal-semiconductor heteronanostructures, and molecularly imprinted polymers (MIPs) nanomaterials to the applications including electro-catalysis, artificial photosynthesis, biochemical sensing, multimodal imaging, drug/gene delivery and photothermo/chemo-therapy.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lu, W., Cui, J., Jiang, R. et al. In-situ wet tearing based subnanometer MoSeS for efficient hydrogen evolution. Sci. China Mater. 60, 929–936 (2017). https://doi.org/10.1007/s40843-017-9112-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9112-4