Abstract
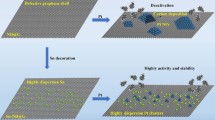
Clean Pt nanoclusters with a diameter of 1.0–2.4 nm, supported on reduced graphene oxide (rGO) nanosheets, were successfully synthesized by simple in situ thermolysis of a Pt-carbonyl complex. The supported Pt nanoclusters are in an electron-deficient state because of the electron transfer between the nanoclusters and the rGO sheets. The as-prepared Pt-1 nm/rGO shows high catalytic activity for the 100% selective hydrogenation of nitrobenzene, with the turnover frequency (TOF) reaching 975.4 h−1 at 25°C and 1 atm. This number is higher than the previously reported value for the heterogeneously catalyzed hydrogenation of nitrobenzene. The proposed process follows a direct hydrogenation mechanism, as is revealed by the analyses of the intermediate products. This work presents a facile and effective synthetic approach for achieving highly efficient nanocatalysts, and can be extended to obtain other metal catalysts with ultra-small sizes and excellent performance.
摘要
本文以羰基铂为前驱体, 采用简便的原位热解方法, 成功地合成了负载在还原氧化石墨烯(rGO)纳米片上的直径为1.0–2.4 nm的清洁Pt纳米团簇. 由于Pt纳米团簇和rGO片之间的电子转移, 所制备的负载Pt纳米团簇处于缺电子状态. 在25°C、1 atm下, 制备的Pt纳米团簇-1 nm/rGO催化剂的硝基苯选择性加氢的转换频率(TOF)达到975.4 h−1. 这个数值是目前硝基苯的非均相催化氢化所报道的活性最高值. 此外, 根据中间产物分析, Pt-1 nm/rGO催化硝基苯加氢的机理是直接氢化. 这项工作在构筑高效催化剂方面提供了一个简单有效的途径, 并且可以进一步扩展制备其他具有超小尺寸和卓越性能的金属催化剂.
Similar content being viewed by others
References
Travis AS. Manufacture and uses of the anilines: a vast array of processes and products. In: Rappoport Z (ed.). The Chemistry of Anilines. West Sussex: JohnWiley & Sons, 2007, 715–782
Nishimura S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis. New York: John Wiley & Sons, 2001, 332–336
Figueras F, Coq B. Hydrogenation and hydrogenolysis of nitro-, nitroso-, azo-, azoxy-and other nitrogen-containing compounds on palladium. J Mol Catal A-Chem, 2001, 173: 223–230
Fang J, Li J, Zhang B, et al. The support effect on the size and catalytic activity of thiolated Au25 nanoclusters as precatalysts. Nanoscale, 2015, 7: 6325–6333
Tomkins P, Gebauer-Henke E, Leitner W, et al. Concurrent hydrogenation of aromatic and nitro groups over carbon-supported ruthenium catalysts. ACS Catal, 2015, 5: 203–209
Mahata A, Rai RK, Choudhuri I, et al. Direct vs. indirect pathway for nitrobenzene reduction reaction on a Ni catalyst surface: a density functional study. Phys Chem Chem Phys, 2014, 16: 26365–26374
Lin W, Cheng H, Ming J, et al. Deactivation of Ni/TiO2 catalyst in the hydrogenation of nitrobenzene in water and improvement in its stability by coating a layer of hydrophobic carbon. J Catal, 2012, 291: 149–154
Sheng B, Hu L, Yu T, et al. Highly-dispersed ultrafine Pt nanoparticles on graphene as effective hydrogenation catalysts. RSC Adv, 2012, 2: 5520–5523
Du W, Chen G, Nie R, et al. Highly dispersed Pt in MIL-101: an efficient catalyst for the hydrogenation of nitroarenes. Catal Commun, 2013, 41: 56–59
Grirrane A, Corma A, García H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science, 2008, 322: 1661–1664
Chong H, Li P, Xiang J, et al. Design of an ultrasmall Au nanocluster–CeO2mesoporous nanocomposite catalyst for nitrobenzene reduction. Nanoscale, 2013, 5: 7622–7628
Jagadeesh RV, Banerjee D, Arockiam PB, et al. Highly selective transfer hydrogenation of functionalised nitroarenes using cobaltbased nanocatalysts. Green Chem, 2015, 17: 898–902
Wisniak J, Klein M. Reduction of nitrobenzene to aniline. Ind Eng Chem Prod Res Dev, 1984, 23: 44–50
Fan X, Zhang G, Zhang F. Multiple roles of graphene in heterogeneous catalysis. Chem Soc Rev, 2015, 44: 3023–3035
Raccichini R, Varzi A, Passerini S, et al. The role of graphene for electrochemical energy storage. Nat Mater, 2014, 14: 271–279
Perreault F, Fonseca de Faria A, Elimelech M. Environmental applications of graphene-based nanomaterials. Chem Soc Rev, 2015, 44: 5861–5896
Ren H, Shao H, Zhang L, et al. Electrodes: a new graphdiyne nanosheet/Pt nanoparticle-based counter electrode material with enhanced catalytic activity for dye-sensitized solar cells. Adv Energ Mater, 2015, 5
Wen P, Gong P, Sun J, et al. Design and synthesis of Ni-MOF/CNT composites and rGO/carbon nitride composites for an asymmetric supercapacitor with high energy and power density. JMater Chem A, 2015, 3: 13874–13883
Sun Z, Zhao Y, Xie Y, et al. The solvent-free selective hydrogenation of nitrobenzene to aniline: an unexpected catalytic activity of ultrafine Pt nanoparticles deposited on carbon nanotubes. Green Chem, 2010, 12: 1007
Nie R, Wang J, Wang L, et al. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon, 2012, 50: 586–596
Lu P, Qiao B, Lu N, et al. Photochemical deposition of highly dispersed Pt nanoparticles on porous CeO2 nanofibers for the water-gas shift reaction. Adv Funct Mater, 2015, 25: 4153–4162
Wu G, Huang H, Chen X, et al. Facile synthesis of clean Pt nanoparticles supported on reduced graphene oxide composites: their growth mechanism and tuning of their methanol electro-catalytic oxidation property. Electrochim Acta, 2013, 111: 779–783
Chen D, Zhao X, Chen S, et al. One-pot fabrication of FePt/reduced graphene oxide composites as highly active and stable electrocatalysts for the oxygen reduction reaction. Carbon, 2014, 68: 755–762
Ikeda S, Ishino S, Harada T, et al. Ligand-free platinum nanoparticles encapsulated in a hollow porous carbon shell as a highly active heterogeneous hydrogenation catalyst. Angew Chem, 2006, 118: 7221–7224
Nakamura J. The effect of a graphene support on the properties of Pt electrode catalysts in fuel cells. Carbon, 2015, 85: 443–444
Galhenage RP, Xie K, Diao W, et al. Platinum–ruthenium bimetallic clusters on graphite: a comparison of vapor deposition and electroless deposition methods. Phys Chem Chem Phys, 2015, 17: 28354–28363
Kwak K, Tang Q, Kim M, et al. Interconversion between superatomic 6-electron and 8-electron configurations of M@Au24(SR)18 clusters (M = Pd, Pt). J Am Chem Soc, 2015, 137: 10833–10840
Imaoka T, Kitazawa H, Chun WJ, et al. Finding the most catalytically active platinum clusters with low atomicity. Angew Chem Int Ed, 2015, 54: 9810–9815
Zhang F, Jiao F, Pan X, et al. Tailoring the oxidation activity of Pt nanoclusters via encapsulation. ACS Catal, 2015, 5: 1381–1385
Hummers Jr. WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc, 1958, 80: 1339–1339
Longoni G, Chini P. Synthesis and chemical characterization of platinum carbonyl dianions [Pt3(CO)6]n 2− (n = ~10,6,5,4,3,2,1). A new series of inorganic oligomers. J Am Chem Soc, 1976, 98: 7225–7231
Qiu JD, Wang GC, Liang RP, et al. Controllable deposition of platinum nanoparticles on graphene as an electrocatalyst for direct methanol fuel cells. J Phys Chem C, 2011, 115: 15639–15645
Tien HW, Huang YL, Yang SY, et al. The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon, 2011, 49: 1550–1560
Liang Y, Zhang H, Zhong H, et al. Preparation and characterization of carbon-supported PtRuIr catalyst with excellent CO-tolerant performance for proton-exchangemembrane fuel cells. J Catal, 2006, 238: 468–476
Ferrari AC, Meyer JC, Scardaci V, et al. Raman spectrum of graphene and graphene layers. Phys Rev Lett, 2006, 97: 187401
Stankovich S, Dikin DA, Piner RD, et al. Synthesis of graphenebased nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 2007, 45: 1558–1565
Rode CV, Vaidya MJ, Jaganathan R, et al. Hydrogenation of nitrobenzene to p-aminophenol in a four-phase reactor: reaction kinetics and mass transfer effects. Chem Eng Sci, 2001, 56: 1299–1304
Wu J, Ong SW, Kang HC, et al. Hydrogen adsorption onmixed platinum and nickel nanoclusters: the influence of cluster composition and graphene support. J Phys Chem C, 2010, 114: 21252–21261
Liang M, Wang X, Liu H, et al. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J Catal, 2008, 255: 335–342
Wang F, Liu J, Xu X. Layered material γ-ZrP supported platinum catalyst for liquid-phase reaction: a highly active and selective catalyst for hydrogenation of the nitro group in para-chloronitrobenzene. Chem Commun, 2008, 2040
Koningsberger D. In situ X-ray absorption spectroscopy as a unique tool for obtaining information on hydrogen binding sites and electronic structure of supported Pt catalysts: towards an understanding of the compensation relation in alkane hydrogenolysis. J Catal, 2003, 216: 178–191
Gelder EA, Jackson SD, Lok CM. The hydrogenation of nitrobenzene to aniline: a new mechanism. Chem Commun, 2005, 522
Corma A, Concepción P, Serna P. A different reaction pathway for the reduction of aromatic nitro compounds on gold catalysts. Angew Chem Int Ed, 2007, 46: 7266–7269
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51402362 and 21471160), Shandong Provincial Natural Science Foundation, China (ZR2014EMQ012 and ZR2016BM12), the Fundamental Research Funds for the Central Universities (15CX08010A,16CX05016 and 16CX05014A), and start-up fund from Tianjin University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guijuan Wei graduated from the School of Chemistry and Materials Science, Ludong University in 2013. She is currently a PhD candidate at China University of Petroleum (UPC) with Prof. Changhua An. Her research interests include the synthesis, characterization, and explorations of efficient catalysts in the fields of clean energy production and environmental purification.
Changhua An received his PhD degree fromthe University of Science and Technology of China (USTC) in 2003 with Prof. Yitai Qian. He has been a full professor of materials science and chemistry since 2013 at UPC. He joined Tianjin University of Technology as a professor in Nov. 2016. His research interests are the synthesis, characterization, and exploration of efficient catalysts in the fields of clean energy production and environmental purification.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wei, G., Zhao, X., An, C. et al. In situ thermolysis of Pt-carbonyl complex to form supported clean Pt nanoclusters with enhanced catalytic performance. Sci. China Mater. 60, 131–140 (2017). https://doi.org/10.1007/s40843-016-5144-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-5144-5