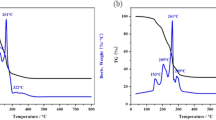
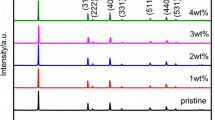
Abstract
Spinel LiMn2−x Si x O4 (x< 1, through substituting Mn4+ with Si4+ in cubic spinel LiMn2O4) was synthesized successfully by a facile sol-gel method. The as-prepared LiMn2−x Si x O4 consisted of pores with large size distribution range from a few nanometers to over 200 nm and possessed specific surface area of 8.76 m2 g−1. Results of X-ray powder diffraction and X-ray photoelectron spectroscopy confirmed that Si atoms entered the host lattice. As a cathode material for rechargeable lithium-ion batteries, spinel LiMn2−x Si x O4 exhibited excellent structural reversibility and integrity during the charging-discharging process. The result indicated that substitution of Mn4+ by Si4+ in spinel LiMn2O4 material effectively alleviated the phase transition caused by Jahn-Teller effect. The initial discharge capacity of the as-prepared spinel LiMn2−x Si x O4 was 147 mA h g−1 over the voltage range of 1.5–4.8 V. However, after 51 cycles, the specific capacity was 88 mA h g−1 with capacity retention of 60 %. More work is needed to understand the effects of substituting Mn4+ by Si4+ and to improve the cyclic stability.
摘要
本文通过溶胶凝胶法成功制备了尖晶石型LiMn2−x Si x O4 (x < 1, 用Si4+替代尖晶石LiMn2O4中的Mn4+). 制备的LiMn2−x Si x O4由几纳米至200纳米的孔组成, 比表面积为8.76 m2 g−1. XRD和XPS的结果表明Si原子进入到晶格中. 作为可充电锂离子电池的正极材料, 充放电过程中 LiMn2−x Si x O4展现出了优异的结构可逆性及完整性. 结果表明用Si4+替代尖晶石LiMn2O4中的Mn4+能有效缓解由Jahn-Teller效应引起的相转变. 尖晶石LiMn2−x Si x O4在1.5–4.8 V的电压窗口下, 首次放电比容量为147 mA h g−1. 51次循环后, 比容量仅为88 mA h g−1, 容量保持率为60%. 因此, 需要进一步了解Si4+替代Mn4+的机理, 从而提高其循环稳定性.
Similar content being viewed by others
References
Massé RC, Uchaker E, Cao G. Beyond Li-ion: electrode materials for sodium-and magnesium-ion batteries. Sci China Mater, 2015, 58: 715–766
Liu C, Neale ZG, Cao G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater Today, 2016, 19: 109–123
Liu C, Massé R, Nan X, et al. A promising cathode for Li-ion batteries: Li3V2(PO4)3. Energy Storage Mater, 2016, 4: 15–58
Tarascon JM. The spinel phase of LiMn2O4 as a cathode in secondary lithium cells. J Electrochem Soc, 1991, 138: 2859–2864
Amatucci G, Tarascon JM. Optimization of insertion compounds such as LiMn2O4 for Li-ion batteries. J Electrochem Soc, 2002, 149: K31–K46
Whittingham MS. Lithium batteries and cathode materials. Chem Rev, 2004, 104: 4271–4302
Chung KY, Kim KB. Investigations into capacity fading as a result of a Jahn–Teller distortion in 4 V LiMn2O4 thin film electrodes. Electrochim Acta, 2004, 49: 3327–3337
Yim H, Kong WY, Yoon SJ, et al. Three-dimensional hemisphere-structured LiSn0.0125Mn1.975O4 thin-film cathodes. Electrochem Commun, 2014, 43: 36–39
Reddy MV, Subba Rao GV, Chowdari BVR. Metal oxides and oxysalts as anode materials for Liion batteries. Chem Rev, 2013, 113: 5364–5457
Xia H, Ragavendran KR, Xie J, et al. Ultrafine LiMn2O4/carbon nanotube nanocomposite with excellent rate capability and cycling stability for lithium-ion batteries. J Power Sources, 2012, 212: 28–34
Amatucci G, Du Pasquier A, Blyr A, et al. The elevated temperature performance of the LiMn2O4/C system: failure and solutions. Electrochim Acta, 1999, 45: 255–271
Li X, Xu Y, Wang C. Suppression of Jahn–Teller distortion of spinel LiMn2O4 cathode. J Alloy Comp, 2009, 479: 310–313
Xiong L, Xu Y, Tao T, et al. Excellent stability of spinel LiMn2O4-based composites for lithium ion batteries. J Mater Chem, 2012, 22: 24563–24568
Gummow RJ, De kock A, Thackeray MM. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells. Solid State Ionics, 1994, 69: 59–67
Guohua L. The spinel phases LiMyMn2-y O4 (M = Co, Cr, Ni) as the cathode for rechargeable lithium batteries. J Electrochem Soc, 1996, 143: 178–182
Capsoni D, Bini M, Chiodelli G, et al. Jahn–Teller transition in Al3+ doped LiMn2O4 spinel. Solid State Commun, 2003, 126: 169–174
Tan CL, Zhou HJ, Li WS, et al. Performance improvement of LiMn2O4 as cathode material for lithium ion battery with bismuth modification. J Power Sources, 2008, 184: 408–413
Shi Y, Zhu S, Zhu C, et al. Synthesis of porous LiFe0.2Mn1.8O4 with high performance for lithium-ion battery. Electrochim Acta, 2015, 154: 17–23
Reddy MV, Cheng HY, Tham JH, et al. Preparation of Li(Ni0.5Mn1.5)O4 by polymer precursor method and its electrochemical properties. Electrochim Acta, 2012, 62: 269–275
Kim DK, Muralidharan P, Lee HW, et al. Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett, 2008, 8: 3948–3952
Li X, Zhou Q, Wang H, et al. Ionothermal synthesis and enhanced electrochemical performance of nanostructure Cr-doped LiMn2O4 for lithium-ion batteries. Ionics, 2015, 21: 1517–1523
Wang S, Yang J, Wu X, et al. Toward high capacity and stable manganese-spinel electrode materials: a case study of Ti-substituted system. J Power Sources, 2014, 245: 570–578
Guan D, Wang Y. Ultrathin surface coatings to enhance cycling stability of LiMn2O4 cathode in lithium-ion batteries. Ionics, 2013, 19: 1–8
Chen R, Knapp M, Yavuz M, et al. Reversible Li+storage in a LiMn-TiO4 spinel and its structural transition mechanisms. J Phys Chem C, 2014, 118: 12608–12616
Wang M, Yang M, Ma L, et al. The high capacity and excellent rate capability of Ti-doped Li2MnSiO4 as a cathode material for Li-ion batteries. RSC Adv, 2015, 5: 1612–1618
Liu XM, Huang ZD, Oh S, et al. Sol–gel synthesis of multiwalled carbon nanotube-LiMn2O4 nanocomposites as cathode materials for Li-ion batteries. J Power Sources, 2010, 195: 4290–4296
Van Horn D. Shannon Effective Ionic Radii, 2001. http://v.web.umkc.edu/vanhornj/shannonradii.htm
Li X, Xu Y, Wang C. Novel approach to preparation of LiMn2O4 core/LiNixMn2-x O4 shell composite. Appl Surface Sci, 2009, 255: 5651–5655
Shin DW, Choi JW, Choi WK, et al. XPS/EXAFS study of cycleability improved LiMn2O4 thin film cathodes prepared by solution deposition. Electrochem Commun, 2009, 11: 695–698
Zhao E, Chen M, Chen D, et al. A versatile coating strategy to highly improve the electrochemical properties of layered oxide LiMO2(M = Ni0.5Mn0.5and Ni1/3Mn1/3Co1/3). ACS Appl Mater Interfaces, 2015, 7: 27096–27105
Zhao X, Reddy MV, Liu H, et al. Nano LiMn2O4 with spherical morphology synthesized by a molten salt method as cathodes for lithium ion batteries. RSC Adv, 2012, 2: 7462–7469
Liu W. Synthesis and electrochemical studies of spinel phase LiMn2O4 cathode materials prepared by the Pechini process. J Electrochem Soc, 1996, 143: 879
Xie X, Su D, Sun B, et al. Synthesis of single-crystalline spinel LiMn2O4 nanorods for lithium-ion batteries with high rate capability and long cycle life. Chem Eur J, 2014, 20: 17125–17131
Hosono E, Kudo T, Honma I, et al. Synthesis of single crystalline spinel LiMn2O4 nanowires for a lithium ion battery with high power density. Nano Lett, 2009, 9: 1045–1051
Xia H, Xia Q, Lin B, et al. Self-standing porous LiMn2O4 nanowall arrays as promising cathodes for advanced 3D microbatteries and flexible lithium-ion batteries. Nano Energy, 2016, 22: 475–482
Zhang X, Yang M, Zhao X, et al. The spinel phase LiMnTiO4 as a potential cathode for rechargeable lithium ion batteries. J Mater Sci-Mater Electron, 2015, 26: 6366–6372
Jiang Q, Xu L, Ma Z, et al. Carbon coated to improve the electrochemical properties of LiMn2O4 cathode material synthesized by the novel acetone hydrothermal method. Appl Phys A, 2015, 119: 1069–1074
Xia Y. Capacity fading on cycling of 4 V Li/LiMn2O4 cells. J Electrochem Soc, 1997, 144: 2593
Esbenshade JL, Fox MD, Gewirth AA. LiMn2O4@Au particles as cathodes for Li-ion batteries. J Electrochemical Soc, 2015, 162: A26–A29
Hwang BM, Kim SJ, Lee YW, et al. Truncated octahedral LiMn2O4 cathode for high-performance lithium-ion batteries. Mater Chem Phys, 2015, 158: 138–143
Author information
Authors and Affiliations
Corresponding authors
Additional information
Min Wang received her BSc degree from Nanjing Tech University. She is currently a PhD candidate at Nanjing Tech University supervised by Prof. Liqun Ma and Prof. Xiaodong Shen. At present, she studies as a visiting student in Prof. Guozhong Cao’s group at the University of Washington. Her research focuses on the electrochemical energy materials for lithium ion batteries.
Liqun Ma received all his degrees (BSc, 1988; MSc, 1991; and PhD, 1995) from Southeast University, China. He then joined the same university as lecturer and associate professor from 1995 to 2000. Meanwhile, He worked as a visiting scientist at Tohoku University in Japan from 1997 to 1998, as a JSPS fellow at Tohoku University from 2000 to 2002, and as a fellow at AIST in Japan from 2003 to 2004. In 2004, he joined Nanjing Tech University as a professor. His research focuses on hydrogen storage materials, metal-matrix composites, damping materials and electrode materials for lithium ion batteries.
Xiaodong Shen is a professor at Nanjing Tech University, where he presently serves as the Chief Scientist of National Basic Research Program of China (973 Program) and the Director of College of Materials Science and Engineering. He received his PhD in 1994 from Nanjing University of Chemical Technology. His current research field is inorganic functional composite materials and advanced energy materials.
Guozhong Cao is Boeing-Steiner Professor of materials science and engineering, professor of chemical engineering, and adjunct professor of mechanical engineering at the University of Washington, and also a professor at Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences and Dalian University of Technology. His current research is focused on chemical processing of nanomaterials for energy related applications including solar cells, rechargeable batteries, supercapacitors, and hydrogen storage.
Rights and permissions
About this article
Cite this article
Wang, M., Yang, M., Zhao, X. et al. Spinel LiMn2−x Si x O4 (x < 1) through Si4+ substitution as a potential cathode material for lithium-ion batteries. Sci. China Mater. 59, 558–566 (2016). https://doi.org/10.1007/s40843-016-5073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-5073-y