Abstract
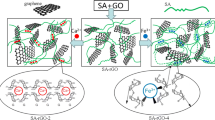
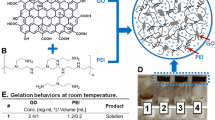
Graphene oxide (GO)-chitosan composite hydrogels were successfully prepared via the self-assembly of chitosan molecules and GO. These as-prepared hydrogels were characterized by different techniques. The morphology of the internal network structure of the nanocomposite hydrogels was investigated. The adsorption capacity results demonstrate that the prepared GObased composite hydrogels can efficiently remove three tested dye molecules, Congo red, methylene blue and Rhodamine B, from wastewater in accordance with the pseudo-second-order model. The dye adsorption capacity of the obtained hydrogels is mainly attributed to the GO sheets, whereas the chitosan molecule was incorporated to facilitate the gelation process of the GO sheets. The present study indicates that the as-prepared composite hydrogels can serve as good adsorbents for wastewater treatment as well as the removal of harmful dyes.
中文摘要
本文通过壳聚糖分子与氧化石墨烯(GO)的自组装成功制备了GO-壳聚糖复合水凝胶, 并对该水凝胶进行了表征. 研究发现复合水凝胶中存在网状微观结构. 吸附性能结果表明制得的基于GO的复合水凝胶可以有效地从废水中去除三种测试染料分子, 同时符合准二阶模型. 该水凝胶的染料吸附能力主要来源于GO片层, 而壳聚糖分子促进了GO片层的凝胶化过程. 目前的研究结果表明制备的复合水凝胶可以作为良好的吸附剂应用于废水处理以及有害染料去除等领域.
Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306: 666–669
Geim AK, Novoselov KS. The rise of graphene. Nat Mater, 2007, 6: 183–191
Li D, Kaner RB. Materials science-graphene-based materials. Science, 2008, 320: 1170–1171
Geim AK. Graphene: status and prospects. Science, 2009, 324: 1530–1534
Huang X, Qi X, Boey F, Zhang H. Graphene-based composites. Chem Soc Rev, 2012, 41: 666–686
Xu YX, Zhao L, Bai H, et al. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis(1-methyl-4-pyridinio) porphyrin and its application for optical detection of cadmium(II) ions. J Am Chem Soc, 2009, 131: 13490–13497
Park S, An J, Jung I, et al. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett, 2009, 9: 1593–1597
Kim J, Cote LJ, Kim F, et al. Graphene oxide sheets at interfaces. J Am Chem Soc, 2010, 132: 8180–8186
Wu S, He Q, Tan C, et al. Graphene-based electrochemical sensors. Small, 2013, 9: 1160–1172
Geng Z, Lin Y, Yu X, et al. Highly efficient dye adsorption and removal: a functional hybrid of reduced graphene oxide–Fe3O4 nanoparticles as an easily regenerative adsorbent. J Mater Chem, 2012, 22: 3527–3535
Wang MY, Zhu W, Zhang DE, et al. CeO2 hollow nanospheres decorated reduced graphene oxide composite for efficient photocatalytic dye-degradation. Mater Lett, 2014, 137: 229–232
Liu F, Chung S, Oh G, Seo TS. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl Mater Interfaces, 2012, 4: 922–927
Sereshti H, Samadi S, Asgari S, Karimi M. Preparation and application of magnetic graphene oxide coated with a modified chitosan pH-sensitive hydrogel: an efficient biocompatible adsorbent for catechin. RSC Adv, 2015, 5: 9396–9404
Wu JH, Chen AP, Qin M, et al. Hierarchical construction of a mechanically stable peptide-graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale, 2015, 7: 1655–1660
Guo H, Jiao T, Zhang Q, et al. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res Lett, 2015, 10: 272
Zhou WW, Ding CY, Jia XT, et al. Self-assembly of Fe2O3/reduced graphene oxide hydrogel for high Li-storage. Mater Res Bull, 2015, 62: 19–23
Adhikari B, Palui G, Banerjee A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter, 2009, 5: 3452–3460
Ray S, Das AK, Banerjee A. pH-responsive, bolaamphiphile-based smart metallo-hydrogels as potential dye-adsorbing agents, water purifier, and vitamin B12 carrier. Chem Mater, 2007, 19: 1633–1639
Adhikari B, Biswas A, Banerjee A. Graphene oxide-based hydrogels to make metal nanoparticle-containing reduced graphene oxide- based functional hybrid hydrogels. ACS Appl Mater Interfaces, 2012, 4: 5472–5482
Biswas A, Banerjee A. Sunlight induced unique morphological transformation in graphene based nanohybrids: appearance of a new tetra-nanohybrid and tuning of functional property of these nanohybrids. Soft Matter, 2015, 11: 4226–4234
Adhikari B, Biswas A, Banerjee A. Graphene oxide-based supramolecular hydrogels for making nanohybrid systems with Au nanoparticles. Langmuir, 2012, 28: 1460–1469
Xu Y, Wu Q, Sun Y, et al. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano, 2010, 4: 7358–7362
Tung VC, Kim J, Cote LJ, Huang J. Sticky interconnect for solution- processed tandem solar cells. J Am Chem Soc, 2011, 133: 9262–9265
Bai H, Li C, Wang X, Shi G. On the gelation of graphene oxide. J Phys Chem C, 2011, 115: 5545–5551
Velmurugan N, Kumar GG, Han SS, et al. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran Polym J, 2009, 18: 383–392
Jiao T, Zhou J, Zhou JX, et al. Synthesis and characterization of chitosan-based Schiff base compounds with aromatic substituent groups. Iran Polym J, 2011, 20: 123–136
Krishna Rao KSV, Madhusudana Rao K, Nagendra Kumar PV, Chung ID. Novel chitosan-based pH sensitive micro-networks for the controlled release of 5-fluorouracil. Iran Polym J, 2010, 19: 265–276
El Achaby M, Essamlali Y, El Miri N, et al. Graphene oxide reinforced chitosan/polyvinylpyrrolidone polymer bio-nanocomposites. J Appl Polym Sci, 2014, 131: 41042
Malini M, Thirumavalavan M, Yang WY, et al. A versatile chitosan/ ZnO nanocomposite with enhanced antimicrobial properties. Int J Biol Macromol, 2015, 80: 121–129
Ordikhani F, Farani MR, Dehghani M, et al. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon, 2015, 84: 91–102
Ardeshirzadeh B, Anaraki NA, Irani M, et al. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mat Sci Eng C Mater, 2015, 48: 384–390
Chowdhuri AR, Tripathy S, Chandra S, et al. A ZnO decorated chitosan-graphene oxide nanocomposite shows significantly enhanced antimicrobial activity with ROS generation. RSC Adv, 2015, 5: 49420–49428
Li D, Muller MB, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol, 2008, 3: 101–105
Chen B, Liu M, Zhang L, et al. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J Mater Chem, 2011, 21: 7736–7741
Chandra V, Kim KS. Highly selective adsorption of Hg2+ by a polypyrrole–reduced graphene oxide composite. Chem Commun, 2011, 47: 3942–3944
Bose S, Kuila T, Uddin ME, et al. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer, 2010, 51: 5921–5928
Konwer S, Boruah R, Dolui SK. Studies on conducting polypyrrole/graphene oxide composites as supercapacitor electrode. J Electron Mater, 2011, 40: 2248–2255
Du X, Guo P, Song HH, Chen XH. Graphene nanosheets as electrode material for electric double-layer capacitors. Electrochim Acta, 2010, 55: 4812–4819
Ferrari AC, Robertson J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B, 2000, 61: 14095–14107
Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS. Raman spectroscopy in graphene. Phys Rep, 2009, 473: 51–87
Kudin KN, Ozbas B, Schniepp HC, et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett, 2008, 8: 36–41
Calizo I, Balandin AA, Bao W, et al. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett, 2007, 7: 2645–2649
Kim KS, Zhao Y, Jang H, et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature, 2009, 457: 706–710
Balashov T, Takács AF, Wulfhekel W, Kirschner J. Magnon excitation with spin-polarized scanning tunneling microscopy. Phys Rev Lett, 2006, 97: 187201
Akhavan O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon, 2015, 81: 158–166
Sharma A, Kumar S, Tripathi B, et al. Aligned CNT/polymer nanocomposite membranes for hydrogen separation. Int J Hydrogen Energy, 2009, 34: 3977–3982
Jiao TF, Wang YJ, Zhang QR, et al. Regulation of substituent groups on morphologies and self-assembly of organogels based on some azobenzene imide derivatives. Nanoscale Res Lett, 2013, 8: 160
Jiao TF, Wang YJ, Gao FQ, et al. Photoresponsive organogel and organized nanostructures of cholesterol imide derivatives with azobenzene substituent groups. Prog Nat Sci, 2012, 22: 64–70
Jiao TF, Gao FQ, Wang YJ, et al. Supramolecular gel and nanostructures of bolaform and trigonal cholesteryl derivatives with different aromatic spacers. Curr Nanosci, 2012, 8: 111–116
Author information
Authors and Affiliations
Corresponding authors
Additional information
Tifeng Jiao is a professor of applied chemistry at the School of Environmental and Chemical Engineering, Yanshan University. He received his PhD degree in physical chemistry in 2006 at the Institute of Chemistry, CAS. His research focuses on the controlled synthesis, self-assembly, and applications of nanocomposites and nanomaterials.
Rights and permissions
About this article
Cite this article
Zhao, H., Jiao, T., Zhang, L. et al. Preparation and adsorption capacity evaluation of graphene oxide-chitosan composite hydrogels. Sci. China Mater. 58, 811–818 (2015). https://doi.org/10.1007/s40843-015-0090-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-015-0090-x