Abstract
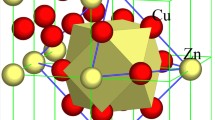
It was recently known that the compositions of industrial alloy specifications generally satisfy simple composition formulas issued from short-range-order structural units in their basic solid solutions. In present work, Cu-Ni face-centered-cubic alloys were further addressed by introducing the cluster-plus-glue-atom model for the short-range-order structural descriptions. Composition formulas covering only the first twelve and the second six neighbor shells in the face-centered-cubic lattice are proposed, [Cu-Cu12](Cu,Ni)6 for the Cu-rich alloys and [Ni-Ni12]Cu6 ~ [Ni-Ni12](Cu5Ni) for Monel alloy, the only Ni-rich alloy specficiation, where the square-bracketed part represents a cuboctahedral cluster. The alloy specification selection is also discussed in terms of valence electron numbers per unit formula. The present work confirms that well-established industrial alloys have simple composition rules that reflect the intrinsic short-range-order local structures in the solid solutions.
概要
工业合金牌号成分一般满足简单的成分式, 我们认为该成分式源自其固溶体中的化学近程有序. 在本文中, 我们通过引入用于描述近程有序结构的团簇与连接原子模型, 进一步详细阐述面心立方结构Cu-Ni合金的工业牌号成分解析. 我们提出的团簇成分式只涉及第一近邻的十二配位立方八面体团簇(方括号部分)和第二近邻的八面体(连接原子部分), 理想满足元素间相互作用: 富铜端的白铜对应于[Cu-Cu12](Cu,Ni)6团簇式, 在富镍端只有蒙乃尔一种工业牌号合金, 用团簇式[Ni-Ni12]Cu6 ~ [Ni-Ni12](Cu5Ni)来解析. 我们还指出, 团簇式对应着特定的价电子总数规律. 本工作进一步证实, 工业合金牌号背后隐藏着体现固溶体近程有序本质的成分规律.
Similar content being viewed by others
References
Hong HL, Wang Q, Dong C, Liaw PK. Understanding the Cu-Zn brass alloys using a short-range-order cluster model: significance of specific compositions of industrial alloys. Sci Rep, 2014, 4: 7065
Xu QJ, Wan ZY, Yin RH, et al. Photoelectrochemical study of influence factors on corrosion resistance of cupronickel B30 in simulated water. Acta Chimica Cinica, 2007, 65: 3–1986
Zheng JT, Zhang SP, Zhou XJ, Zeng E, Hu ZZ. Comparative study of erosion-corrosion of B10 and B30 cupronickel. Equip Enviorn Eng, 2010, 7: 3–43
Dey GK, Mukhopadhyay P. Precipitation in the Ni-Cu-base alloy monel K-500. Mater Sci Eng, 1986, 84: 3–189
Han C, Zhou JT, Fan ZK. Effect of heat treatment on microstructure and hardness of monel alloy containing silicon. Trans Mater Heat Treat, 2009, 30: 3–109
Wang HR, Tian XL, Teng XY, et al. Short-range and medium-range order in liquid Cu-Ni alloy. Chin Phys Lett, 2002, 19: 3–235
Claus H, Tranchita CJ. Magentism and atomic short-range order in V-Fe and Cu-Ni. J Phys Colloques, 1978, 39: C6-858-C6-859
Lohofer G, Brillo J, Egry I. Thermophysical properties of undercooled liquid Cu-Ni alloys. Int J Thermophys, 2004, 25: 3–1550
Deutsches KL. Chemical Colourings of Copper and Copper Alloys. Sydney: Copper and Brass Information Centre, 1965
Davis JR. ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys. Ohio: ASM international, 2000
Reinhard L, Schönfeld B, Kostorz G, Bührer W. Short range order in a-brass. Phys Rev B, 1990, 44: 3–1734
Rossiter P L. Effects of co-existing atomic and magnetic clustering on electrical resistivity Cu-Ni alloys. J Phys F Metal Phy, 1981, 11: 3–2118
Bessiere M, Lefebvre S, Calvayrac Y. X-ray diffraction study of short-range order in a disordered Au3Cu alloy. Acta Cryst, 1983, B39: 145–153
Aalders J, van Dijk C, Radelaar S. Neutron scattering study of shortrange clustering in CuNi(Fe) alloys. J Phy F Met Phys, 1984, 14: 3–2815
Chen ZY, Dai GT. Comparative study of dispersion relations of onedimensional diatomic chain lattice vibration with different neighbor interactions. J China Three Gorges Univ, 2010, 32: 3–104
Cowley JM. An approximate theory of order in alloys. Phys Rev, 1950, 77: 3–675
Stolz UK, Arpshofen I, Sommer F, Predel B. Determination of the enthalpy of mixing of liquid alloys using a high-temperature mixing calorimeter. J Phase Equilib, 1993, 14: 3–478
Liu HB, Chen KY, Hu ZQ. Application of the embedded-atom method to liquid binary Cu-Ni alloys. J Mater Sci Technol, 1997, 13: 3–122
Vrijen J. Netherlands Energy Research Foundation ECN Petten Report ECN-31, 1977
Dong C, Wang Q, Qiang JB, et al. From clusters to phase diagrams: composition rules of quasicrystals and bulk metallic glasses. J Phys D Appl Phys, 2007, 40: R273–R291
Han G, Qiang JB, Li FW, et al. The e/a values of ideal metallic glasses in relation to cluster formulae. Acta Mater, 2011, 59: 3–5926
Hume-Rothery W, Raynor G V. The Structure of Metals and Alloys. 4th ed. London: Institute of Metals, 1962
Xie YQ, Qu ZT, Ma LY, Xie ZC. The electronic structure and thermal electricity, corrosion resistance and strength of Cu-Ni alloys. J Funct Mater, 1977, 42–49
Gong XG, Kumar V. Enhanced stability of magic cluster: a case study of icosahedral Al12X, X=B, Al, Ga, C, Si, Ge, Ti, As. Phys Rev Lett, 1993, 70: 3–2081
Knight WD, Clemenger K, Heer WA, et al. Electronic shell structure and abundances of sodium clusters. Phys Rev Lett, 1984, 52: 3–2144
Chou MY, Cohen ML. Electronic shell structure in simple metal clusters. Phys Rev Lett, 1986, 113A: 420–424
Mizutani U, Sato H, Inukai M, Zijlstra ES. e/a Determination for 4d- and 5d-transition metal elements and their intermetallic compounds with Mg, Al, Zn, Cd and In. Philos Mag, 2013, 93: 3–3390
Zhang J, Wang Q, Wang YM, et al. Revelation of solid solubility limit Fe/Ni=1/12 in corrosion-resistant Cu–Ni alloys and relevant cluster model. J Mater Res, 2010, 25: 3–336
Wang Q, Zha QF, Liu EX, et al. Composition design of high strength martensitic precipitation hardening stainless steels based on a cluster model. Acta Metall Sin, 2012, 48: 3–1206
Wang Q, Ji CJ, Wang YM, Qiang JB, Don C. β-Ti alloys with low Young’s moduli interpreted by cluster-plus-glue-atom model. Metall Mater Trans A, 2013, 44:1872–1879
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Lian Hong received her BSc degree in Physics from Anshan Normal College in 2000. Now she is a PhD candidate under the supervision of Prof. Chuang Dong at Dalian University of Technology (DUT). Her research interest focuses on solid solution alloys.
Qing Wang received her BSc degree from Shandong University in 1999, MSc degree in Materials Science from Lanzhou University of Technology in 2002 (supervisor: Prof. Jianhong Chen), and PhD degree in Materials Science from DUT in 2002 (supervisor: Prof. Chuang Dong) in 2005. Since then, she has been working at DUT as assistant and associate professor. During this period, she did a one-year visiting scholar at the University of Tennessee (2013–2014). Her main research interests focus on the alloy design in multi-component systems, including low-E Ti alloys, conductivity Cu alloys, high-performance stainless steels, etc.
Chuang Dong received his BSc degree in Dalian Institute of Technology and PhD degree at Institute of Polytechnique de Lorraine (France). In 1996, he obtained the Outstanding Young Scholar Funding from NSFC. He became the director of the State Key Laboratory of Materials Modification by Laser, Ion, and Electron Beams. In 2005, he was appointed the Cheung Kong Professor. His research interests include materials design and surface modification. He serves as editorial advisory board member of SCIENCE CHINA Materials, Acta Metallurgica Sinica, Chinese Heat treatment, Functional Materials, Chinese Surface Techniques, and Proceedings of Dalian University of Technology.
Rights and permissions
About this article
Cite this article
Hong, H., Wang, Q. & Dong, C. Composition formulas of Cu-Ni industrial alloy specifications. Sci. China Mater. 58, 355–362 (2015). https://doi.org/10.1007/s40843-015-0049-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-015-0049-y