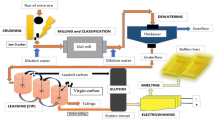
Abstract
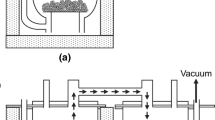
The oxygen content of the copper powders significantly affects the properties of copper, particularly its morphology and electric conductivity. Moreover, the oxygen content depends on the chosen synthesis method and variation of the process parameters. In this study, a sustainable oxygen-free copper powder electrolysis method was exploited to synthesize the copper powder by using wastes as initial materials. The anode and cathode copper scrap plates were used as raw materials. To accomplish this, the effect of the variation of the process parameters including current density, concentration of sulfuric acid, and copper ions was studied. Heat treatment was carried out at 600 °C for 90 min under H2 atmosphere for deoxidization. Additionally, the effect of the mechanical reduction through carbon bed on deoxidization (carbothermic reduction) was evaluated prior to the heat treatment under a hydrogen atmosphere. The oxygen content of achieved powders was measured by using the ONH analysis method based on the ASTM E 2575 standard method. The minimum measured oxygen content was found to be 179 ppm in the optimized condition of 0.3 A.cm−2, 140 g.l−1 of concentration of acid sulfuric, and 5 g.l−1 concentration of copper ions. This low oxygen content is attributed to the spherical and flake-shaped morphology of obtained copper powders. The size, morphology, and crystallization of the obtained copper powders were evaluated by scanning electron microscopy (SEM) and EDS. Results revealed that with the variation of the process parameters, the size of the powders was changed between 4.03 and 11.18 µm.
Graphical Abstract





Similar content being viewed by others
References
Mehrabani SAN, Tabrizi AT, Aghajani H, Pourbagheri H (2020) Corrosion behavior of SHS-produced Cu–Ti–B composites. Int J Self-Propag High-Temp Synth 29(3):167–172. https://doi.org/10.3103/S1061386220030061
Sorkhe YA, Aghajani H, Taghizadeh Tabrizi A (2014) Mechanical alloying and sintering of nanostructured TiO2 reinforced copper composite and its characterization. Mater Des 58:168–174. https://doi.org/10.1016/j.matdes.2014.01.040
Li Q, Lan Z, Chun J, Lian S, Wen R, Ma X (2021) Fabrication and capillary characterization of multi-scale micro-grooved wicks with sintered copper powder. Int Commun Heat Mass Transf 121:105123. https://doi.org/10.1016/j.icheatmasstransfer.2021.105123
Abdel-Aziz MH (2011) Hydrometallurgy Production of copper powder from wastewater containing CuSO4 and alcoholic additives in a modified stirred tank reactor by cementation. Hydrometallurgy 109(1–2):161–167. https://doi.org/10.1016/j.hydromet.2011.06.007
Agrawal A, Kumar V, Pandey BD, Sahu KK (2006) A comprehensive review on the hydro metallurgical process for the production of nickel and copper powders by hydrogen reduction. Mater Res Bull 41:879–892. https://doi.org/10.1016/j.materresbull.2005.09.028
Park YS et al (2016) Fabrication of dendritic silver-coated copper powders by galvanic displacement reaction and their thermal stability against oxidation. Appl Surf Sci 389:865–873. https://doi.org/10.1016/j.apsusc.2016.08.008
Qi Z et al (2021) Scalable fabrication of high activity nanoporous copper powders for electrochemical CO2 reduction via ball milling and dealloying. J CO2 Util 45:101454. https://doi.org/10.1016/j.jcou.2021.101454
Morvan A et al (2019) Powder processing methodology for fabrication of copper/graphite composite materials with enhanced thermal properties. Compos Part A Appl Sci Manuf 124:105474. https://doi.org/10.1016/j.compositesa.2019.105474
Liang Q et al (2023) Electrolyte circulation : Metal recovery from waste printed circuit boards of mobile phones by alkaline slurry electrolysis. J Clean Prod 409:137223. https://doi.org/10.1016/j.jclepro.2023.137223
Liang YU, Ding ZY, Liu SJ, Shu WF, He YN (2017) Effects of additives on zinc electrodeposition from alkaline zincate solution. Trans Nonferr Met Soc China 27(7):1656–1664. https://doi.org/10.1016/S1003-6326(17)60188-2
Bisang JM (2021) Intensification electrochemical production of cobalt powder by using a modified hydrocyclone with ultrasonic assistance. Chem Eng Proces Process Intensif. https://doi.org/10.1016/j.cep.2021.108560
Wranglen G (1950) Electrodeposition of metal powders. J Electrochem Soc. https://doi.org/10.1149/1.2777895
Pavlović MG, Pavlović LJ, Maksimović VM, Nikolićand ND, Popov KI (2010) Characterization and morphology of copper powder particles as a function of different electrolytic regimes. Int J Electrochem Sci 5(12):1862–1878
Nikolić ND, Avramović L, Ivanović ER, Maksimović VM, Baščarević Z, Ignjatović N (2019) Comparative morphological and crystallographic analysis of copper powders obtained under different electrolysis conditions. Trans Nonferr Met Soc China 29(6):1275–1284. https://doi.org/10.1016/S1003-6326(19)65034-X
Sekar R (2017) Synergistic effect of additives on electrodeposition of copper from cyanide-free electrolytes and its structural and morphological characteristics. Trans Nonferr Met Soc China 27(7):1665–1676. https://doi.org/10.1016/S1003-6326(17)60189-4
Kartal L, Timur S (2018) Electrolytic production of Cu–Ni alloys in CaCl2-Cu2S-NiS molten salt. Trans Nonferr Met Soc China 28(10):2143–2150. https://doi.org/10.1016/S1003-6326(18)64859-9
Shokobayev NM, Nurtazina AE, Kholkin OS, Abilmagzhanov AZ, Ivanov NS, Adelbaev IE (2022) Results in engineering obtaining of copper powder by method of a vortex electrolysis from acid sulphate electrolytes. Results Eng 16:100604. https://doi.org/10.1016/j.rineng.2022.100604
Zhao L, Zhang X, Deng T, Jiang J (2018) Develop an effective oxygen removal method for copper powder. Adv Powder Technol 29(8):1904–1912. https://doi.org/10.1016/j.apt.2018.05.001
Hossein Sehhat M, Perez-Palomino D, Wiedemeier C, Cullom T, Newkirk JW (2023) Characterization of virgin, re-used, and oxygen-reduced copper powders processed by the plasma spheroidization process. Adv Powder Technol 34(1):103885. https://doi.org/10.1016/j.apt.2022.103885
Nie C et al (2023) Eco-friendly strategy for advanced recycling waste copper from spent lithium-ion batteries: preparation of micro-nano copper powder. Sep Purif Technol 322:124277. https://doi.org/10.1016/j.seppur.2023.124277
Çuvalcı O, Varol T, Akçay SB, Güler O, Çanakçı A (2023) Effect of ball mill time and wet pre-milling on the fabrication of Ti powders by recycling Ti machining chips by planetary milling. Powder Technol 426:118637. https://doi.org/10.1016/j.powtec.2023.118637
Avramović L et al (2018) Correlation between crystal structure and morphology of potentiostatically electrodeposited silver dendritic nanostructures. Trans Nonferr Met Soc China 28(9):1903–1912. https://doi.org/10.1016/S1003-6326(18)64835-6
Fan K, Jiang W, Ruiz-Hervias J, Bausin C, Feng W, Zhou H, Bueno S, Yao P (2021) Effect of Al2TiO5 contect and sintering temperature on the microstructure and residual stress of Al2O3-Al2TiO5 ceramic composites. Materials 14(24):7624. https://doi.org/10.3390/ma14247624
Nyathi TM, Fischer N, York PE, Claeys M (2017) Effect of crystallite size on the performance and phase transformation of Co3O4/Al2O3 catalysts during CO-PrOx – an in situ study. Faraday Discuss. https://doi.org/10.1039/c6fd00217j
Author information
Authors and Affiliations
Contributions
SE: Investigation, Methodology, Formal Analysis, HA: Supervision, Writing-reviewing and editing, ATT: Methodology, Writing-Editing Original Draft.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Veena Sahajwalla.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emami, S., Aghajani, H. & Tabrizi, A.T. Sustainable Oxygen-Free Copper Powder Production Method from Wastes. J. Sustain. Metall. 9, 1803–1809 (2023). https://doi.org/10.1007/s40831-023-00766-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00766-2