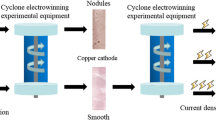
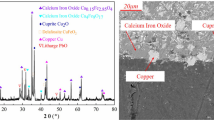
Abstract
In this paper, the valuable metal copper was recovered from the leaching solution of copper–cadmium slag by cyclone electrowinning technology. During the cyclone electrowinning process, it was found that adding sodium 4-hydroxyphenylsulfonate could make the surface of cathode copper obtained by electrodeposition change benignly, make the surface of cathode copper smooth, and reduce the appearance of depressions and cracks. The effects of different sodium 4-hydroxyphenylsulfonate concentrations and different current densities on cathode copper were investigated: When the concentration range of sodium 4-hydroxyphenylsulfonate was 0 ~ 10 mg/L, the current efficiency decreased from 97.73 to 92.19%, and the deposition rate decreased from 38.95 to 36.74 μm/h; When the current density was in the range of 200 ~ 400 m/A2, the highest current efficiency was 92.59%, and the deposition rate increased linearly. The existence of sodium 4-hydroxyphenylsulfonate could make copper particles smaller in the electrodeposition process and inhibit the crystallization of different sizes; at high current densities, the cathode copper produces an increasingly pronounced nodular bulge, and although the deposition rate of metal ions increases linearly, the deposit morphology tends to deteriorate (rough and protrusion on the surface). Choosing proper concentration of sodium 4-hydroxyphenylsulfonate and current density could make cathode copper better in morphology and purity. Reasonable recovery of copper and cadmium slag could effectively reduce the environmental pollution caused by smelting waste and realize the secondary utilization of smelting waste.
Graphical Abstract










Similar content being viewed by others
References
Tian H, Guo Z, Pan J et al (2021) Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour Conserv Recycl 168:105366. https://doi.org/10.1016/j.resconrec.2020.105366
Zhang C, Wang A, Jiang H et al (2020) Environmental activity and ecological assessment of heavy metals in the reductive leaching residue from zinc hydrometallurgy industry. Trans Indian Inst Met 73:1755–1761. https://doi.org/10.1007/s12666-020-01991-z
Liu H, Wang S, Fu L et al (2022) Mechanism and kinetics analysis of valuable metals leaching from copper-cadmium slag assisted by ultrasound cavitation. J Clean Prod 379:134775. https://doi.org/10.1016/j.jclepro.2022.134775
Đukić-Ćosić D, Baralić K, Javorac D et al (2020) An overview of molecular mechanisms in cadmium toxicity. Curr Opin Toxicol 19:56–62. https://doi.org/10.1016/j.cotox.2019.12.002
Burke F, Hamza S, Naseem S et al (2016) Impact of cadmium polluted groundwater on human health. SAGE Open 6:215824401663440. https://doi.org/10.1177/2158244016634409
Zhou Y, Wang L, Xiao T et al (2020) Legacy of multiple heavy metal(loid)s contamination and ecological risks in farmland soils from a historical artisanal zinc smelting area. Sci Total Environ 720:137541. https://doi.org/10.1016/j.scitotenv.2020.137541
Liu A, Ma Y, Gunawardena JMA et al (2018) Heavy metals transport pathways: the importance of atmospheric pollution contributing to stormwater pollution. Ecotoxicol Environ Saf 164:696–703. https://doi.org/10.1016/j.ecoenv.2018.08.072
Li B, Wang X, Wei Y et al (2018) Extraction of copper from copper and cadmium residues of zinc hydrometallurgy by oxidation acid leaching and cyclone electrowinning. Miner Eng 128:247–253. https://doi.org/10.1016/j.mineng.2018.09.007
Li M, Zhang Y, Wang X-H et al (2021) Extraction of copper, zinc and cadmium from copper–cadmium-bearing slag by oxidative acid leaching process. Rare Met 40:1–10. https://doi.org/10.1007/s12598-016-0759-7
Xu H, Li B, Wei Y, Wang H (2020) Extracting of copper from simulated leaching solution of copper-cadmium residues by cyclone electrowinning technology. Hydrometallurgy 194:105298. https://doi.org/10.1016/j.hydromet.2020.105298
Hong J, Xu Z, Li D et al (2020) A pilot study: efficient electrowinning of tellurium from alkaline solution by cyclone electrowinning technology. Hydrometallurgy 196:105429. https://doi.org/10.1016/j.hydromet.2020.105429
Wang Y, Xue Y, Su J et al (2018) Efficient electrochemical recovery of dilute selenium by cyclone electrowinning. Hydrometallurgy 179:232–237. https://doi.org/10.1016/j.hydromet.2018.05.019
Kim Y, Cho H, Lee H et al (2002) Electrowinning of palladium using a modified cyclone reactor. J Appl Electrochem 32:1235–1239. https://doi.org/10.1023/A:1021667015212
Xu Z, Guo X, Tian Q et al (2020) Electrodeposition of tellurium from alkaline solution by cyclone electrowinning. Hydrometallurgy 193:105316. https://doi.org/10.1016/j.hydromet.2020.105316
Guo X, Qin H, Tian Q, Li D (2020) Recovery of metals from waste printed circuit boards by selective leaching combined with cyclone electrowinning process. J Hazard Mater 384:121355. https://doi.org/10.1016/j.jhazmat.2019.121355
Lafouresse MC, Heard PJ, Schwarzacher W (2007) Surface roughness analysis of electrodeposited Cu. Electrochim Acta 53:229–232. https://doi.org/10.1016/j.electacta.2007.05.068
Winand R (1998) Contribution to the study of copper electrocrystallization in view of industrial applications—submicroscopic and macroscopic considerations. Electrochim Acta 43:2925–2932. https://doi.org/10.1016/S0013-4686(98)00033-4
Wang Y, Li B, Xu H, Guo J (2021) Effect of Cd2+ on electrodeposition of copper in cyclone electrodeposition. Metals (Basel) 11:529. https://doi.org/10.3390/met11040529
Wang Y, Li B, Wei Y, Wang H (2022) Effect of Zn2+ on the extraction of copper by cyclone electrowinning from simulated copper-containing electrolyte. Sep Purif Technol 282:120014. https://doi.org/10.1016/j.seppur.2021.120014
Yang W, Wang Y, Li B et al (2023) Effect of Fe3+ on electrowinning of copper by CE. Miner Eng 191:107942. https://doi.org/10.1016/j.mineng.2022.107942
Garduño-Corvera G, Garfias-Ayala FJ, Garfias-Vazquez FJ (2011) Effect of some additives on the zinc electrodeposition process. ECS Trans 36:267–273. https://doi.org/10.1149/1.3660620
Sung M, Kim HC, Lim T, Kim JJ (2017) Effects of organic additives on grain growth in electrodeposited Cu thin film during self-annealing. J Electrochem Soc 164:D805–D809. https://doi.org/10.1149/2.0481713jes
Lou W, Cai W, Li P et al (2018) Additives-assisted electrodeposition of fine spherical copper powder from sulfuric acid solution. Powder Technol 326:84–88. https://doi.org/10.1016/j.powtec.2017.12.060
Collet T, Wouters B, Hallemans N et al (2023) The time-varying effect of thiourea on the copper electroplating process with industrial copper concentrations. Electrochim Acta 437:141412. https://doi.org/10.1016/j.electacta.2022.141412
Fabian CP, Ridd MJ, Sheehan ME (2007) Assessment of activated polyacrylamide and guar as organic additives in copper electrodeposition. Hydrometallurgy 86:44–55. https://doi.org/10.1016/j.hydromet.2006.11.002
Ni J, Han K, Yu M, Zhang C (2017) The influence of sodium citrate and potassium sodium tartrate compound additives on copper electrodeposition. Int J Electrochem Sci 12:6874–6884. https://doi.org/10.20964/2017.07.57
Nkuna EH, Popoola API (2019) Effect of chloride electrolyte additive on the quality of electrorefined copper cathode. Procedia Manuf 35:789–794. https://doi.org/10.1016/j.promfg.2019.06.024
Wu M, Xu C, Wang W et al (2018) Effects of sodium hydroxyethyl sulfonate on improving the ductility of the copper films deposited by electroless plating from high-speed plating baths. ChemistrySelect 3:5082–5086. https://doi.org/10.1002/slct.201702385
Chen Z, Yang X, Fu Y (2020) Influence of sodium propargyl sulfonate on electrodeposition of Fe–Co alloy. J Alloys Compd 826:154167. https://doi.org/10.1016/j.jallcom.2020.154167
Chen H-M, Parulekar SJ, Zdunek A (2008) Interactions of chloride and 3-mercapto-1-propanesulfonic acid in acidic copper sulfate electrolyte. J Electrochem Soc 155:D349. https://doi.org/10.1149/1.2844405
Li S, Dai M, Wu Y et al (2022) Resource utilization of electroplating wastewater: obstacles and solutions. Environ Sci (Camb) 8:484–509. https://doi.org/10.1039/D1EW00712B
Cheng X, Li YF, Huang GJ et al (2019) The effects of current density on microstructure and properties of electrolytic copper foils. Mater Sci Forum 944:205–211. https://doi.org/10.4028/www.scientific.net/MSF.944.205
Davidson R, Verma A, Santos D et al (2020) Mapping mechanisms and growth regimes of magnesium electrodeposition at high current densities. Mater Horiz 7:843–854. https://doi.org/10.1039/C9MH01367A
Schmidt R, Gaida J (2017) Cuprous ion mass transport limitations during copper electrodeposition. ChemElectroChem 4:1849–1851. https://doi.org/10.1002/celc.201700208
Acknowledgements
Financial support for this study was supplied from the National Natural Science Foundation of China (Project Nos. 51764035) and the Natural Science Foundation of Yunnan province (Project Nos. 2018FB089).
Funding
National Natural Science Foundation of China, 51764035, Bo Li, Natural Science Foundation of Yunnan Province, 2018FB089, Bo Li.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, L., Li, B., Wei, Y. et al. Smoothing Enhancement of Sodium 4-Hydroxyphenylsulfonate on Cathode Copper from Simulated Leachate of Copper–Cadmium Slag by Cyclone Electrowinning. J. Sustain. Metall. 9, 1732–1743 (2023). https://doi.org/10.1007/s40831-023-00761-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00761-7