Abstract
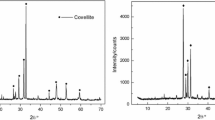
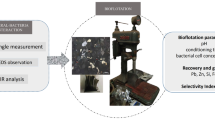
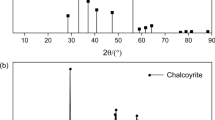
Flotation separation of chalcopyrite/pyrite mixtures under Mg(OH)2 depression effectively limits pyrite entrainment into Cu concentrates. However, the Cu concentrate is still negatively affected by the low selectivity of depression by Mg(OH)2 and unavoidable floatability activation of pyrite by surface Cu contamination via galvanic effects. In this study, ethylenediaminetetraacetic acid (EDTA) was used to control the effects of Mg(OH)2 depression on the recoveries of chalcopyrite and pyrite, and to restrict Cu activation in pyrite flotation. Compared with EDTA-free systems, a system with the optimal EDTA dosage (5 × 10−5 M EDTA for 1 × 10−2 M Mg) gave better separation of Mg(OH)2-depressed chalcopyrite and pyrite, with recoveries varying from 63.5 to 94.0% (chalcopyrite) and from 49.9 to 47.5% (pyrite), respectively. Zeta potential measurements and micro-zone X-ray photoelectron spectroscopy (μ-XPS) indicated that Mg(OH)2 detachment was achieved because the surface electropositivity of the Mg(OH)2 colloid decreased because of EDTA chemisorption on its surface as Mg(EDTA)2−. The thermal field-emission scanning electron microscopy-energy dispersive spectroscopy and μ-XPS analysis showed that the preferential removal of surface Cu decelerated Mg(OH)2 removal from the pyrite surface. The results of this study show that moderate EDTA dosages potentially enable effective separation of chalcopyrite and pyrite. EDTA modification is anticipated to optimize the effect of Mg(OH)2 depression on the flotation separation of chalcopyrite and pyrite. This would facilitate cleaner production in Cu pyrometallurgical process.
Graphical Abstract









Similar content being viewed by others
References
Lotter NO, Bradshaw DJ (2010) The formulation and use of mixed collectors in sulphide flotation. Miner Eng 23:945–951. https://doi.org/10.1016/j.mineng.2010.03.011
Sillitoe RH (2010) Porphyry copper systems. Econ Geol 105:3–41. https://doi.org/10.2113/gsecongeo.105.1.3
Cheng H, Liu Q, Huang M, Zhang S, Frost RL (2013) Application of TG-FTIR to study SO2 evolved during the thermal decomposition of coal-derived pyrite. Thermochim Acta 555:1–6. https://doi.org/10.1016/j.tca.2012.12.025
Dimitrijević M, Kostov A, Tasić V, Milosević N (2009) Influence of pyrometallurgical Cu production on the environment. J Hazard Mater 164:892–899. https://doi.org/10.1016/j.jhazmat.2008.08.099
Ji G, Liao Y, Wu Y, Xi J, Liu Q (2022) A review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite. J Sustain Metall 8:964–977. https://doi.org/10.1007/s40831-022-00561-5
Lee RLJ, Chen X, Peng Y (2022) Flotation performance of chalcopyrite in the presence of an elevated pyrite proportion. Miner Eng 177:107387. https://doi.org/10.1016/j.mineng.2021.107387
Bulatovic SM (2007) Handbook of flotation reagents: chemistry, theory and practice: flotation of sulfide ores, vol 1. Elsevier, Amsterdam
Fuerstenau MC, Jameson GJ, Yoon RH (2007). Froth flotation: a century of innovation. SME
Moslemi H, Gharabaghi M (2017) A review on electrochemical behavior of pyrite in the froth flotation process. J Ind Eng Chem 47:1–18. https://doi.org/10.1016/j.jiec.2016.12.012
Chandra AP, Gerson AR (2009) A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv Colloid Interface Sci 145:97–110. https://doi.org/10.1016/j.cis.2008.09.001
Hirajima T, Suyantara GPW, Ichikawa O, Elmahdy AM, Miki H, Sasaki K (2016) Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner Eng 96:83–93. https://doi.org/10.1016/j.mineng.2016.06.023
Suyantara GPW, Hirajima T, Miki H, Sasaki K (2018) Floatability of molybdenite and chalcopyrite in artificial seawater. Miner Eng 115:117–130. https://doi.org/10.1016/j.mineng.2017.10.004
Yang X, Li Y, Fan R, Duan W, Huang L, Xiao Q (2022) Separation mechanism of chalcopyrite and pyrite due to H2O2 treatment in low-alkaline seawater flotation system. Miner Eng 176:107356. https://doi.org/10.1016/j.mineng.2021.107356
Chen Y, Zhang G, Shi Q, Yang S, Liu D, Wang M (2020) Utilization of trisodium phosphate to eliminate the adverse effect of Mg2+ on the flotation of pyrite. Miner Eng 150:106281. https://doi.org/10.1016/j.mineng.2020.106281
Fuchida S, Xue J, Ishida S, Tokoro C (2022) Kinetic investigation of initial oxidative dissolution of pyrite in alkaline media (pH 9–12) and influence of Ca and Mg: a fundamental study for pyrite depression in froth flotation. J Sustain Metall 8:732–741. https://doi.org/10.1007/s40831-022-00521-z
Xue J, Fuchida S, Ishida S, Tokoro C (2022) Insight on exogenous calcium/magnesium in weakening pyrite floatability with prolonged pre-oxidation: localized and concomitant secondary minerals and their depression characteristics. Minerals 12:115. https://doi.org/10.3390/min12020115
Li W, Li Y, Xie S, Duan W, Chen W (2021) Roles and influences of kerosene on chalcopyrite flotation in MgCl2 solution: EDLVO and DFT approaches. Minerals 12:48. https://doi.org/10.3390/min12010048
Li W, Li Y, Xiao Q, Wei Z, Song S (2018) The influencing mechanisms of sodium hexametaphosphate on chalcopyrite flotation in the presence of MgCl2 and CaCl2. Minerals 8:150. https://doi.org/10.3390/min8040150
Li Y, Zhu H, Li W, Zhu Y (2019) A fundamental study of chalcopyrite flotation in sea water using sodium silicate. Miner Eng 139:105862. https://doi.org/10.1016/j.mineng.2019.105862
Peng Y, Li Y, Li W, Fang X, Liu C, Fan R (2020) Elimination of adverse effects of seawater on molybdenite flotation using sodium silicate. Miner Eng 146:106108. https://doi.org/10.1016/j.mineng.2019.106108
Bodine MW Jr, Fernalld TH (1973) EDTA dissolution of gypsum, anhydrite, and Ca–Mg carbonates. J Sediment Res 43(4):1152–1156
Bulatovic SM (1999) Use of organic polymers in the flotation of polymetallic ores: a review. Miner Eng 12:341–354. https://doi.org/10.1016/S0892-6875(99)00015-1
Mu Y, Peng Y, Lauten RA (2016) The depression of pyrite in selective flotation by different reagent systems—a literature review. Miner Eng 96:143–156. https://doi.org/10.1016/j.mineng.2016.06.018
Aghazadeh S, Mousavinezhad SK, Gharabaghi M (2015) Chemical and colloidal aspects of collectorless flotation behavior of sulfide and non-sulfide minerals. Adv Colloid Interface Sci 225:203–217. https://doi.org/10.1016/j.cis.2015.09.007
Tao D, Wang Y, Li L (2018) An electrochemical study of surface oxidation and collectorless flotation of pyrite. Int J Electrochem Sci 13:5971–5982. https://doi.org/10.20964/2018.06.32
Lelis DF, Fernandes Lima RM, Rocha GM, Leão VA (2022) Effect of magnesium species on cationic flotation of quartz from hematite. Miner Process Extr Metall Rev 43:339–345. https://doi.org/10.1080/08827508.2020.1864362
Zhang W, Honaker RQ (2018) Flotation of monazite in the presence of calcite part II: enhanced separation performance using sodium silicate and EDTA. Miner Eng 127:318–328. https://doi.org/10.1016/j.mineng.2018.01.042
Wang J, Liu Q, Zeng H (2013) Understanding copper activation and xanthate adsorption on sphalerite by time-of-flight secondary ion mass spectrometry, X-ray photoelectron spectroscopy, and in situ scanning electrochemical microscopy. J Phys Chem C 117:20089–20097. https://doi.org/10.1021/jp407795k
Wu J, Ma W, Wang X, Jiao F, Qin W (2020) The effect of galvanic interaction between chalcopyrite and pyrite on the surface chemistry and collector adsorption: flotation and DFT study. Colloid Surf A 607:125377. https://doi.org/10.1016/j.colsurfa.2020.125377
Lascelles D, Finch JA, Sui C (2003) Depressant action of Ca and Mg on flotation of Cu activated sphalerite. Can Metall Q 42:133–140. https://doi.org/10.1179/cmq.2003.42.2.133
Agorhom EA, Skinner W, Zanin M (2014) Diethylenetriamine depression of Cu-activated pyrite hydrophobised by xanthate. Miner Eng 57:36–42. https://doi.org/10.1016/j.mineng.2013.12.010
Agorhom EA, Skinner W, Zanin M (2015) Post-regrind selective depression of pyrite in pyritic copper–gold flotation using aeration and diethylenetriamine. Miner Eng 72:36–46. https://doi.org/10.1016/j.mineng.2014.11.019
Aikawa K, Ito M, Kusano A, Park I, Oki T, Takahashi T, Furuya H, Hiroyoshi N (2021) Flotation of seafloor massive sulfide ores: combination of surface cleaning and deactivation of lead-activated sphalerite to improve the separation efficiency of chalcopyrite and sphalerite. Metals 11:253. https://doi.org/10.3390/met11020253
Shabani GM, Afradi A, Obeid RA, Abdullah FSA, Alnassar YS, Hameed NM, Abbood SK (2023) Alkali metal-doped borospherenes M@ C4B32 (M = K, Na, and Li) as a highly efficient alternative for the drug delivery. J Mol Model 29:147. https://doi.org/10.1007/s00894-023-05548-x
Moses CO, Herman JS (1991) Pyrite oxidation at circumneutral pH. Geochim Cosmochim Acta 55:471–482. https://doi.org/10.1016/0016-7037(91)90005-P
Owusu C, e Abreu SB, Skinner W, Addai-Mensah J, Zanin M (2014) The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures. Miner Eng 55:87–95. https://doi.org/10.1016/j.mineng.2013.09.018
Hirajima T, Miki H, Suyantara GPW, Matsuoka H, Elmahdy AM, Sasaki K, Imaizumi Y, Kuroiwa S (2017) Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner Eng 100:83–92. https://doi.org/10.1016/j.mineng.2016.10.007
Zanin M, Lambert H, du Plessis CA (2019) Lime use and functionality in sulphide mineral flotation: a review. Miner Eng 143:105922. https://doi.org/10.1016/j.mineng.2019.105922
Qiu Z, Liu G, Liu Q, Zhong H (2016) Understanding the roles of high salinity in inhibiting the molybdenite flotation. Colloid Surf A 509:123–129. https://doi.org/10.1016/j.colsurfa.2016.08.059
Smart RSC (1991) Surface layers in base metal sulphide flotation. Miner Eng 4:891–909. https://doi.org/10.1016/0892-6875(91)90072-4
Schmid RW, Reilley CN (1956) A rapid electrochemical method for the determination of metal chelate stability constants. J Am Chem Soc 78:5513–5518
Vaghefinazari B, Snihirova D, Wang C, Wang L, Deng M, Höche D, Lamaka SV, Zheludkevich ML (2022) Exploring the effect of sodium salt of ethylenediaminetetraacetic acid as an electrolyte additive on electrochemical behavior of a commercially pure Mg in primary Mg-air batteries. J Power Sources 527:231176. https://doi.org/10.1016/j.jpowsour.2022.231176
Voigt S, Szargan R, Suoninen E (1994) Interaction of copper(II) ions with pyrite and its influence on ethyl xanthate adsorption. Surf Interface Anal 21:526–536. https://doi.org/10.1002/sia.740210804
Weisener C, Gerson A (2000) Cu (II) adsorption mechanism on pyrite: an XAFS and XPS study. Surf Interface Anal 30:454–458. https://doi.org/10.1002/1096-9918(200008)30:1%3c454::AID-SIA807%3e3.0.CO;2-1
Ejtemaei M, Nguyen AV (2017) Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner Eng 100:223–232. https://doi.org/10.1016/j.mineng.2016.11.005
Amaral LF, Oliveira IR, Bonadia P, Salomão R, Pandolfelli VC (2011) Chelants to inhibit magnesia (MgO) hydration. Ceram Int 37:1537–1542. https://doi.org/10.1016/j.ceramint.2011.01.030
Zhang C, Wang S, Cao ZF, Zhong H (2018) Kinetics and mechanism of one-step reductive leaching of manganese oxide ores by EDTA/EDTA–2Na. Physicochem Probl Miner Process 54:858–867. https://doi.org/10.5277/ppmp1887
Xiao Y, Wang T, Qiu G, Zhang K, Xue C, Li B (2020) Synthesis of EDTA-bridged CdS/g-C3N4 heterostructure photocatalyst with enhanced performance for photoredox reactions. J Colloid Interface Sci 577:459–470. https://doi.org/10.1016/j.jcis.2020.05.099
Acknowledgements
The authors sincerely thank the Kagami Memorial Research Institute for Materials Science and Technology, and the Materials Characterization Central Laboratory, Waseda University for assistance with FE-SEM–EDS and μ-XPS analysis, respectively. Jifeng Xue gratefully acknowledges the financial support of the China Scholarship Council. We thank Helen McPherson, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Hiromichi Takebe.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, J., Oyama, K., Fuchida, S. et al. Improving Flotation Separation of Mg(OH)2-Depressed Chalcopyrite and Pyrite by EDTA Modification: A Promising Method for Sustainable Cu Production. J. Sustain. Metall. 9, 1647–1659 (2023). https://doi.org/10.1007/s40831-023-00754-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00754-6