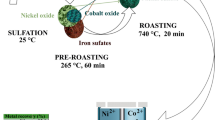
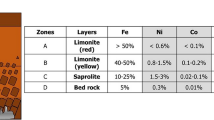
Abstract
The sulfation roasting leaching method has been developed for the recovery of nickel from laterite ore because it offers greater flexibility, and requires less energy compared to acid leaching and smelting processes. However, the previous studies found that the mineral carriers from saprolite ore affected the recovery of nickel and cobalt. To address this issue, alkali salts such as Na2SO4, K2SO4, (NH4)2SO4 and NaCl have been introduced into the sulfation roasting leaching process due to their high reactivity with nickel and cobalt at elevated temperatures. The current study aimed to investigate the effect of adding various alkali salts on the recovery of nickel and cobalt from saprolite ore in sulfation roasting leaching process. Under optimized condition of sulfation with 0.8 mL H2SO4/g ore mixed with 40 wt% moisture followed by roasting at 700 °C for 30 min and leaching at 80 °C for 30 min, the results showed that the highest recovery of 90% nickel was achieved by addition of 5 wt% Na2SO4. Other alkali salts exhibited positive effects on the increased recovery of nickel during sulfation roasting leaching process. Based on the results, the mechanism of alkali salts on increase in the recovery of nickel during this process was proposed in terms of detailed characterization by X-Ray Diffraction and thermal analyses. The addition of alkali salts played important role in promoting the sulfation of metal oxide by forming liquid pyrosulfate as an intermediate phase at elevated temperatures, which penetrated into sulphated ore, and provided more sulfuric trioxide gas to form more sulfates. This approach may serve as an alternative means of enhancing the formation of sulfates in the sulfation roasting leaching process.
Graphical Abstract
Illustrated mechanism of adding Na2SO4 and K2SO4 to sulfation roasting process
















Similar content being viewed by others
References
Dalvi AD, Bacon WG, Osborne RC (2004) The past and the future of nickel laterites. PDAC 2004 International Conference Trade Show and Investors Exchange, Toronto, 1–27
Meshram P, Abhilash P, Pandey BD (2019) Advanced review on extraction of nickel from primary and secondary sources. Min Proc Extractive Met Rev 40(3):157–193. https://doi.org/10.1080/08827508.2018.1514300
Zevgolis EN, Daskalakis KA (2021) The nickel production method from laterites and the Greek ferronickel production among them. Mat Proceedings 5:104. https://doi.org/10.3390/materproc2021005104
Oxley A, Barcza N (2013) Hydro-pyro integration in the processing of nickel laterites. Miner Eng 54:2–13. https://doi.org/10.1016/j.mineng.2013.02.012
Crundwell FK, Moats MS, Ramachandran V, Robinson TG, Davenport WG (2011) Chapter 1—Overview. Extractive metallurgy of nickel, cobalt and platinum group metals. Elsevier, Amsterdam. https://doi.org/10.1016/C2009-0-63541-8
Ribeiro PPM, Dos Santos ID, Neumann R, Fernandes A, Dutra AJB (2021) Roasting and leaching behavior of nickel laterite ore. Metall Mater Trans B 52B:1739–1754. https://doi.org/10.1007/s11663-021-02141-6
Sun Q, Cheng H, Mei X, Liu Y, Li G, Xu Q, Lu X (2020) Efficient synchronous extraction of nickel, copper, and cobalt from low-nickel matte by sulfation roasting-water leaching process. Sci Rep 10:9916. https://doi.org/10.1038/s41598-020-66894-x
Guo X, Li D, Park KH, Tian Q, Wu Z (2009) Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process. Hydrometallurgy 99(3–4):144–150. https://doi.org/10.1016/j.hydromet.2009.07.0120
Xu Y, Xie Y, Yan L, Yang R (2005) A new method for recovering valuable metals from low-grade nickeliferous oxide ores. Hydrometallurgy 80(4):280–285. https://doi.org/10.1016/j.hydromet.2005.08.007
Kar BB, Swamy YV (2000) Some aspects of nickel extraction from chromitiferous overburden by sulphatization roasting. Min Eng 13(14–15):1635–1640. https://doi.org/10.1016/S0892-6875(00)00147-3
Swamy YV, Kar BB, Mohanty JK (2003) Physico-chemical characterization and sulphatization roasting of low-grade nickeliferous laterites. Hydrometallurgy 69(1–3):89–98. https://doi.org/10.1016/S0304-386X(03)00027-6
Prasad S, Pandey BD (1998) Alternative processes for treatment of chalcopyrite—A review. Miner Eng 11:763–781. https://doi.org/10.1016/S0892-6875(98)00061-2
Dong J, Wei Y, Zhou S, Li B, Yang Y, Mclean A (2018) The effect of additives on extraction of Ni, Fe and Co from nickel laterites ores. JOM 70:2365–2377. https://doi.org/10.1007/s11837-018-3032-8
Li G, Cheng H, Chen S, Lu X, Xu Q, Lu C (2018) Mechanism of Na2SO4 promoting nickel extraction from sulfide concentrates by sulfation roasting-water leaching. Metall Mater Trans B 49B:1136–1148. https://doi.org/10.1007/s11663-018-1214-y
Ford AN, Meehan BJ, Tariq SA (1982) Molten potassium pyrosulfate: reactions of metals. Aust J Chem 35(2):437–441. https://doi.org/10.1071/CH9820437
Ammasi A (2020) Effect of heating rate on decomposition temperature of goethite ore. Trans Indian Inst Met 73(1):93–98. https://doi.org/10.1007/s12666-019-01806-w
Yang X, Gao L, Wu Y, Chen Y, Tong L (2022) Extraction of magnesium and nickel from nickel-rich serpentine with sulfation roasting and water leaching. Metals 12(2):318. https://doi.org/10.3390/met12020318
Wang Z, Yang W, Liu H, Jin H, Chen H, Su K, Tu Y, Wang W (2019) Thermochemical behavior of three sulfates (CaSO4, K2SO4 and Na2SO4) blended with cement raw materials (CaO–SiO2–Al2O3–Fe2O3) at high temperature. J Anal Appl Pyrolisis 142:104617–104627. https://doi.org/10.1016/j.jaap.2019.05.006
Chen TM, Chen RH (1994) High-temperature structual phase transition of K2SO4 and K2SeO4 crsytals studied by X-ray diffraction. J Solid State Chem 111:338–342. https://doi.org/10.1006/jssc.1994.1236
Kiyoura R, Urano K (1970) Mechanism, kinetics, and equilibrium of thermal decomposition of ammonium sulfate. Ind Eng Process Des Develop 9:489–494. https://doi.org/10.1021/i260036a001
Zhou S, Wei Y, Li B, Wang H, Ma B, Wang C (2016) Mechanism of sodium chloride in promoting reduction of high magnesium low-nickel oxide ore. Sci Rep 6:29061. https://doi.org/10.1038/srep29061
De Vries KJ, Gellings PJ (1969) Thermal decomposition of potassium and sodium pyrosulfate. J Inorg Nucl Chem 31:1307–1313. https://doi.org/10.1016/0022-1902(69)80241-1
Liu C, Lindsay WT Jr (1972) Thermodynamics of sodium chloride solutions at high temperature. J Solut Chem 1(1):45–68. https://doi.org/10.1007/BF00648416
Liu K, Chen Q, Hu H (2009) Comparative leaching of minerals by sulphuric acid in a Chinese ferruginous nickel laterite ore. Hydrometallurgy 98:281–286. https://doi.org/10.1016/j.hydromet.2009.05.015
Abdel-Aal EA, Rashad MM (2004) Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid. Hydrometallurgy 74:189–194. https://doi.org/10.1016/j.hydromet.2004.03.005
Hubli RC, Mittra J, Suri AK (1997) Reduction-dissolution of cobalt oxide in acid media: a kinetic study. Hydrometallurgy 44:125–134. https://doi.org/10.1016/S0304-386X(96)00036-9
Skeaff JM, Espelund AW (1973) An E.M.F. method for the determination of sulphate-oxide equilibria results for the Mg, Mn, Fe, Ni, Cu and Zn systems. Can Metall Q 12:445–454. https://doi.org/10.1179/cmq.1973.12.4.445
Onal MAR, Borra CR, Guo M, Blanpain B (2017) Recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J Sustain Metall 1:199–215. https://doi.org/10.1007/s40831-015-0021-9
Tagawa H (1984) Thermal decomposition temperatures of metal sulfates. Thermochim Acta 80:23–33. https://doi.org/10.1016/0040-6031(84)87181-6
Scheidema MN, Taskinen P (2011) Decomposition thermodynamics of magnesium sulfate. Ind Eng Chem Res 50:9550–9556. https://doi.org/10.1021/ie102554f
Ranjani VS, James AP Jr, Edward PF, Ming-Shing S, Angela LM (1999) Decomposition of The sulfates of copper, iron(II), iron(III), nickel. Appl Surf Sci 152:219–236. https://doi.org/10.1016/S0169-4332(99)00319-0
Porus M, Labbez C, Maroni P, Borkovec M (2011) Adsorption of monovalent and divalent cations on planar water-silica interfaces studied by optical reflectivity and Monte Carlo simulations. J Chem Phys 135(6):064701. https://doi.org/10.1063/1.3622858
Ribeiro PPM, de Souza LCM, Neumann R, dos Santos ID, Dutra AJB (2020) Nickel and cobalt losses from laterite ore after the sulfation-roasting-leaching processing. J Mat Res Tech 9(6):12404–12415. https://doi.org/10.1016/j.jmrt.2020.08.082
Anderson AB, Debnath NC (1983) Mechanism for forming sodium pyrosulfate from sodium chloride, sulfur dioxide and oxygen. J Phys Chem 87:1938–1941
Hu Z, Zhang L, Sang Q, Wang X, Wang Z, Tan H (2016) Mechanism investigation on the sulfation of condensed sodium chloride at 523–1023 K. Clean Coal Tech Sustain Dev. https://doi.org/10.1007/978-981-10-2023-0_12
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Il Sohn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hariyanto, R.K.S., Tomas da Rocha , L., Kim, SJ. et al. Influence of Alkali Salts on Extraction of Nickel From Serpentine-Rich Ore Through Sulfation Roasting Leaching Process. J. Sustain. Metall. 9, 1636–1646 (2023). https://doi.org/10.1007/s40831-023-00753-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00753-7