Abstract
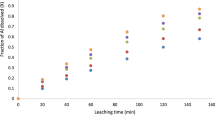
Indonesia has many mineral resources that have not been properly processed and utilized, such as ilmenite. Banten ilmenite is one of the Indonesia’s potential ilmenite deposits. Iron and titanium are the primary components of Banten ilmenite, with titanium being processed into titanium dioxide (TiO2). Direct leaching is one of the ilmenite processing methods that can be done on a large scale. The purpose of this research is to investigate and to develop an appropriate mathematical model for the direct leaching process of Banten ilmenite using hydrochloric acid. Ilmenite that passed through the 250–149 µm sieve was leached with 360 mL of hydrochloric acid at a stirring speed of 300 rpm and a solid/liquid (S/L) ratio of 1:4. The different acid concentrations used were 3 M, 7 M, and 11 M, with process temperature variations of 30 °C, 60 °C, and 90 °C. At 5, 10, 15, 20, 30, 90, and 150 min, 5 mL samples of the solution were taken. The results indicate that the concentration of hydrochloric acid and the temperature had an effect on the Ti recovery results. Ti recovery increases as the concentration of hydrochloric acid and process temperature increase. The highest Ti recovery was 84.52%, achieved at a concentration of 11 M at 90 °C for 150 min. The Lump model is used to simulate ilmenite leaching kinetics, in which the leaching process is controlled by the ash layer's diffusion step and chemical reactions. The Lump model is a mathematical model that can illustrate the phenomenon of ilmenite Banten direct leaching.
Graphical Abstract











Similar content being viewed by others
References
Woodruff L, Bedinger G (2013) Titanium─light, strong, and white. USGS Numbered Ser. https://doi.org/10.3133/fs20133059
U.S. Geological Survey (2016) Mineral commodity summaries 2016: U.S. Geological Survey. p 202. https://doi.org/10.3133/70140094
Van Gosen BS, Bleiwas DI, Bedinger GM, Ellefsen KJ, Shah AK (2016) Coastal deposits of heavy mineral sands: global significance and US resources. Miner Eng 68(10):36–43
Aristanti Y, Supriyatna YI, Masduki NP, Soepriyanto S (2019) Effect of calcination temperature on the characteristics of TiO2 synthesized from ilmenite and its applications for photocatalysis. IOP Conf Ser: Mater Sci Eng 478:012019. https://doi.org/10.1088/1757-899x/478/1/012019
Ministry of Energy and Mineral Resources, Central Mineral, Coal, and Geothermal Resources (2021) Balance sheet of mineral, coal, and geothermal resources and reserves for 2021. pp 15–16
Supriyatna Yayat Iman, Astuti Widi, Sumardi Slamet, Sudibyo Sudibyo, Prasetya Agus, Ginting Lavita Indriyani, Irmawati Yuyun, Asri Nining Sumawati, Petrus Himawan Tri Bayu Murti (2021) Correlation of nano titanium dioxide synthesis and the mineralogical characterization of ilmenite ore as raw material. Int J Technol 12(4):749–759
Aristanti Y, Supriyatna YI, Masduki NP, Soepriyanto S (2018) Decomposition of banten ilmenite by caustic fusion process for TiO2 photocatalytic applications. IOP Conf Ser: Mater Sci Eng 285:012005. https://doi.org/10.1088/1757-899x/285/1/012005
Tsuchida H, Narita E, Takeuchi H, Adachi M, Okabe T (1982) Manufacture of high pure titanium (IV) oxide by the chloride process : I. Kinetic study on leaching ilmenite ore in concentrated hydrochloric acid solution. Bull Chem Soc Japan 55(6):1934–1938
Mohanty SP, Smith KA (1993) Alkali metal catalysis of carbothermic reaction of ilmenite. Trans Inst Min Metall Sect C: Min Process Extr Metall 102:C163–C173
Mackey TS (1994) Upgrading ilmenite into a high-grade synthetic rutile. JOM. https://doi.org/10.1007/BF03220676
Mahmoud YD, Georges JK (1997) Processing titanium and lithium for reduced-cost application. JOM 49(6):20–27
Gázquez MJ, Bolívar JP, Garcia-Tenorio R, Vaca F (2014) A review of the production cycle of titanium dioxide pigment. Mater Sci Appl 5(7):441–458. https://doi.org/10.4236/msa.2014.57048
Nayl AA, Aly HW (2009) Acid leaching of ilmenite decomposed by KOH. Hydrometallurgy 97(1–2):86–93. https://doi.org/10.1016/j.hydromet.2009.01.011
Adams R (2018) TiO2─the search for stability. Informa Mineral Sands. Informa, Perth
Mccoy D, Coetzee B, Keegal M, Bender E (2011) Titanium feedstocks─opaque quality requirements. Eight international heavy minerals conference 2011. Melbourne, The Australasian Institute of Mining and Metallurgy, pp 197–204
Zhang S, Nicol MJ (2010) Kinetics of the dissolution of ilmenite in sulfuric acid solutions under reducing conditions. Hydrometallurgy 103(1–4):196–204. https://doi.org/10.1016/j.hydromet.2010.03.019
Jabit NA, Senanayake G (2018) Characterization and leaching kinetics of ilmenite in hydrochloric acid solution for titanium dioxide production. IOP Conf Ser: J Phys. https://doi.org/10.1088/1742-6596/1082/1/012089
Ramadan AM, Farghaly MM, Fathy W, Ahmed MM (2016) Leaching and kinetics studies on processing of Abu-Ghalaga ilmenite ore. Int Res J Eng Technol (IRJET) 03(10):46–53
Wanta KC, Astuti W, Perdana I, Petrus HTBM (2020) Kinetic study in atmospheric pressure organic acid leaching: shrinking core model versus lump model. Minerals 10(7):613. https://doi.org/10.3390/min10070613
Geankoplis CJ (1993) Transport processes and unit operation, 3rd edn. Prentice-Hall International Editions, Upper Saddle River, p 385
McCabe WL, Smith JC, Harriott P (1993) Unit operations of chemical engineering, 5th edn. McGraw-Hill International Editions, Singapore, p 650
Berk Z (2018) Chapter 3—heat and mass transfer, basic principles. Food process engineering and technology. Academic Press, Cambridge, pp 79–126
Liang Y (2018) Diffusion in encyclopedia of geochemistry. Springer International Publising, Berlin/Heidelberg, pp 1–13
Fogler HS (2006) Elements of chemical reaction engineering, 4th edn. Prentice Hall Professional Technical Reference, Upper Saddle River, p 83
Supriyatna YI, Sumardi S, Astuti W, Nainggolan AN, Ismail AW, Petrus HTBM, Prasetya A (2020) Characterization and a preliminary study of TiO2 synthesis from Lampung Iron Sand. Key Eng Mater 849:113–118. https://doi.org/10.4028/www.scientific.net/
Hollitt MJ, Kisler JP, Raahauge B (2002) The Comalco bauxite activation process, sixth alumina quality workshop, Brisbane, pp. 115–122
Lahiri A, Kumari EJ, Jha A (2006) Kinetic studies on the soda-ash roasting of titanoferous ores for the extraction of TiO2. Proceedings of the Sohn international symposium on advanced processing of metals and materials. Vol. 1: Thermo and physicochemical principles: non-ferrous high-temperature processing, pp. 115–123.
Olanipekun E (1999) A Kinetic study of the leaching of a Nigerian ilmenite ore by hydrochloric acid. Hydrometallurgy 53(1):1–10. https://doi.org/10.1016/S0304-386X(99)00028-6
Nabivanets B, Kudritskaya LN (1967) A study on the polymerization of titanium (IV) in hydrochloric acid solutions. Russ J Inorg Chem 12(5):616
Levenspiel O (1999) Chemical reaction engineering, 3rd edn. Wiley, New York. https://doi.org/10.1021/ie990488g
Probstein RF (1989) Physicochemical hydrodynamics─an introduction, 1st edn. Butterworth Publishers, Massachusetts
Wen C (1968) Noncatalytic heterogeneous solid fluid reaction models. Ind Eng Chem Res 47(6):459–471. https://doi.org/10.1021/ie50705a007
Ramos-Cano J, González-Zamarripa G, Carrillo-Pedroza FR, Soria-Aguilar MDJ, Hurtado-Macías A, Cano-Vielma A (2016) Kinetics and statistical analysis of nickel leaching from spent catalyst in nitric acid solution. Int J Miner Process 148:41–47. https://doi.org/10.1016/j.minpro.2016.01.006
Zhu X, Li W, Guan X (2015) Kinetics of titanium leaching with citric acid in sulfuric acid from red mud. Trans Nonferrous Metals Soc China 25(9):3139–3145. https://doi.org/10.1016/S1003-6326(15)63944-9
Acknowledgements
We would like to thank the Department of Chemical Engineering (Sustainable Mineral Processing Research Group), Faculty of Engineering, Gadjah Mada University and Research Center for Mining Technology-National Research and Innovation Agency (PRTPB-BRIN) for the facility and the financial support to complete this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
The contributing editor for this article was Grace Ofori-Sarpong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anggraeni, V.M.P., Supriyatna, Y.I., Astuti, W. et al. Ilmenite Sand Direct Leaching Kinetics in Hydrochloric Acid Solution. J. Sustain. Metall. 9, 1578–1588 (2023). https://doi.org/10.1007/s40831-023-00749-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00749-3