Abstract
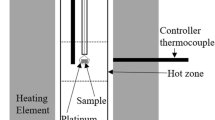
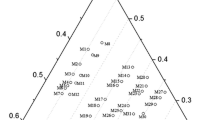
It has been proved that Cr2O3 is favorable to promote the crystallization of perovskite in the Selective Crystallization and Phase Separation (SCPS) process. However, the related thermodynamic data of equilibrium phase relations was quite limited. In the present work, the equilibrium phase relations of CaO-SiO2-TiO2 system with the addition of 5 wt% Cr2O3 were experimentally carried out using the high temperature equilibration-quenching technique followed by X-Ray Diffraction (XRD), Scanning Electron Microscopy-Energy Dispersive X-Ray Spectroscopy (SEM–EDS), as well as thermodynamic calculation. More attention has been focused on the influence of 5 wt% Cr2O3 addition on the liquid-perovskite two-phase coexisting region. The results indicated that the crystallization of perovskite was promoted by Cr2O3 addition by expanding the primary phase of perovskite toward higher SiO2 areas. Meanwhile, distinct discrepancies were found when the present experimental results were compared with the data from the literature and the isotherms calculated by FactSage, indicating that the present results are not only meaningful for the improvement of the SCPS process, but also important for the optimization of titania related oxide thermodynamic database.
Graphical Abstract







Similar content being viewed by others
References
Ma H, Jiao K, Zhang J, Zong Y, Zhang J, Meng S (2021) Viscosity of CaO-MgO-Al2O3-SiO2-TiO2-FeO slag with varying TiO2 content: the effect of crystallization on viscosity abrupt behavior. Ceram Int 47:17445–17454
Chen D, Zhao L, Liu Y, Qi T, Wang J, Wang L (2013) A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J Hazard Mater 244–245:588–595
Shi J, Qiu Y, Yu B, Xie X, Dong J, Hou C, Li J, Liu C (2022) Titanium extraction from titania-bearing blast furnace slag: a review. JOM 74:654–667
Zhang L, Zhang LN, Wang MY, Li GQ, Sui ZT (2007) Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner Eng 20:684–693
Yang Z, Yang F, Yi M, Xiang L (2021) Estimation of reaction heat in Ti-bearing blast furnace slag-sulfuric acid system based on mechanical mixture model. Mining Metall Explor 38:1247–1252
Hu M, Wei R, Hu M, Wen L, Ying F (2018) Nonisothermal carbothermal reduction kinetics of titanium-bearing blast furnace slag. JOM 70:1443–1448
Zhen YL, Zhang GH, Chou KC (2016) Carbothermic reduction of titanium-bearing blast furnace slag. High Temp Mat Pr-isr 35:309–319
Zhen YL, Zhang GH, Chou KC (2016) Mechanism and kinetics of the carbothermic reduction of titanium-bearing blast furnace slag. Metall Res Technol 113:507
Zhang GH, Wang KF (2017) Preparation of Ti5Si3 by silicothermic reduction of titanium-bearing blast furnace slag. Can Metall Quart 57:80–88
Huang QY, Lv XW, Huang R, Song JJ (2013) Preparation of Ti-Si-Al alloy by aluminothermic reduction of TiO2 bearing blast furnace slag. Can Metall Quart 52:413–421
He S, Peng T, Sun H (2019) Titanium recovery from Ti-bearing blast furnace slag by alkali calcination and acidolysis. JOM 71:3196–3201
Bian ZZ, Feng YL, Li HR (2020) Extraction of valuable metals from Ti-bearing blast furnace slag using ammonium sulfate pressurized pyrolysis–acid leaching processes. T Nonferr Metal Soc 30:2836–2847
He S, Sun H, Tan DG, Peng T (2016) Recovery of titanium compounds from Ti-enriched product of alkali melting Ti-bearing blast furnace slag by dilute sulfuric acid leaching. Procedia Environ Sci 31:977–984
Jiang T, Dong HG, Guo YF, Li GH, Yang YB (2013) Study on leaching Ti from Ti bearing blast furnace slag by sulphuric acid. Miner Process Extr M 119:33–38
Ma GQ, Cheng M (2014) Technological study of titanium slag production from titanium-bearing blast furnace slag. Adv Mater Res 962–965:793–796
Lei XF, Chen C, Li X, Xue XX, Yang H (2014) Study on the preparation process of photocatalysts by the acidolysis of high titanium slag with hydrochloric acid. Appl Mech Mater 662:3–6
Zheng F, Chen F, Guo Y, Jiang T, Travyanov AY, Qiu G (2016) Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag. JOM 68:1476–1484
Zheng F, Guo Y, Qiu G, Chen F, Wang S, Sui Y, Jiang T, Yang L (2018) A novel process for preparation of titanium dioxide from Ti-bearing electric furnace slag: NH4HF2-HF leaching and hydrolyzing process. J Hazard Mater 344:490–498
Wang L, Liu W, Hu J, Liu Q, Yue H, Liang B, Zhang G, Luo D, Xie H, Li C (2018) Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3. Chinese J Chem Eng 26:583–592
Pu Z, Jiao H, Mi Z, Wang M, Jiao S (2020) Selective extraction of titanium from Ti-bearing slag via the enhanced depolarization effect of liquid copper cathode. J Energy Chem 42:43–48
Hu M, Wei R, Qu Z, Yin F, Xu Y, Deng Q (2016) Preparation of TiC by carbothermal reduction in vacuum and acid leaching using blast furnace slag bearing titania. Green Process Synth 5(2):195–203
Zhang PX, Sui ZT, Yamauchi C (1998) Precipitation selectivity of boron compounds from slags. Acta Metall 47:1337–1344
Fan Y, Shibata E, Iizuka A, Nakamura T (2015) Crystallization behavior of copper smelter slag during molten oxidation. Metall Mater Trans B 46:2158–2164
Shi J, Qiu Y, Yu B, Zhao F, Ma W, Li J, Liu C (2021) Review and perspective of the vanadium extraction techniques from converter vanadium-bearing slag. Chem Ind Eng Prog 12:5281–5292
Wang MY, Zhang LN, Zhang L, Sui ZT, Tu GF (2006) Selective enrichment of TiO2 and precipitation behavior of perovskite phase in titania bearing slag. T Nonferr Metal Soc 16:421–425
Qiu G, Chen L, Zhu J, Lv X, Bai C (2015) Effect of Cr2O3 addition on viscosity and structure of Ti-bearing blast furnace slag. ISIJ Int 55:1367–1376
Shi J, Qiu Y, Wan X, Yu B, Chen M, Zhao F, Li J, Liu C, Taskinen P (2022) Equilibrium phase relations of the CaO-SiO2-Ti3O5 system at 1400 degrees C and a p(O-2) of 10(-16) atm. JOM 74:668–675
Jongejan A, Wilkins AL (1971) A Re-examination of the system CaO-TiO2 at liquidus temperatures. J Common Metals 20:273–279
Gong W, Wu L, Navrotsky A (2018) Combined experimental and computational investigation of thermodynamics and phase equilibria in the CaO-TiO2 system. J Am Ceram Soc 101:1361–1370
Kirillova SA, Almjashev VI, Gusarov VV (2011) Phase relationships in the SiO2-TiO2 system. Russ J Inorg Chem 56:1464
Shindo I (1980) Determination of the phase diagram by the slow cooling float zone method: the system MgO-TiO2. J Cryst Growth 50(4):839–851
Park YJ, Kim WY, Kang YB (2021) Phase equilibria of Al2O3-TiO system under various oxygen partial pressure: emphasis on stability of Al2TiO5–Ti3O5 pseudobrookite solid solution. J Eur Ceram Soc 41:7362–7374
Wan X, Shi J, Klemettinen L, Chen M, Taskinen P, Jokilaakso A (2020) Equilibrium phase relations of CaO-SiO2-TiO2 system at 1400°C and oxygen partial pressure of 10–10 atm. J Alloys Compd 847:156472
Qiu Y, Shi J, Yu B, Hou C, Dong J, Li S, Zhai Y, Li J, Liu C (2022) Phase equilibria of MgO-Al2O3-TiO2 system at 1600°C in air: emphasis on pseudobrookite and spinel solid solution phases. J Am Ceram Soc. https://doi.org/10.2139/ssrn.4063777
Qiu Y, Shi J, Hou C, Dong J, Zhai Y, Li K, Yan S, Yu K, Li J, Liu C (2022) 1700°C isothermal phase diagram of the MgO-Al2O3-TiO2 system in air related to pseudobrookite and spinel ceramics. JOM 75:1982–1992
Ilatovskaia M, Fabrichnaya O (2019) Thermodynamic assessment of the Al2O3-MgO-TiO2 system. J Alloy Compd 790:1137–1148
Chen M, Wan X, Taskinen P, Sukhomlinov D, Shi J, Michallik R, Jokilaakso A (2022) Phase equilibria in TiO2-rich part of the MgO-CaO-TiO2 system at 1500–1600°C. Ceram Int 48:20116–20125
Chen M, Wan X, Shi J, Taskinen P, Jokilaakso A (2022) Experimental study on the phase relations of the SiO2-MgO-TiO2 system in air at 1500°C. JOM 74:676–688
Wan X, Shi J, Qiu Y, Chen M, Li J, Liu C, Taskinen P, Jokilaakso A (2021) The effect of 15 wt% Al2O3 addition on the equilibrium phase relations of CaO-SiO2-TiO2 system at 1400°C in air. Ceram Int 47:24802–24808
Wan X, Chen M, Qiu Y, Shi J, Li J, Liu C, Taskinen P, Jokilaakso A (2021) Influence of manganese oxide on the liquid-perovskite equilibrium in the CaO-SiO2-TiO2 system at 1400°C in air. Ceram Int 47:11176–11182
Kirschen M, DeCapitani C (1999) Experimental determination and computation of the liquid miscibility gap in the system CaO-MgO-SiO2-TiO2. J Phase Equilib 20:593–611
Wang Z, Zhu Q, Sun H (2019) Phase equilibria in the TiO2-rich part of the TiO2-CaO-SiO2-10 wt%-Al2O3-5wt%-MgO system at 1773K. Metall Mater Trans B 50:357–366
Li Y, Qiu Y, Shi J, Zhang B, Meng F, Li J, Liu C (2021) Equilibrium phase relations of a SiO2-Al2O3-FeOx System with 10wt% CaO addition for the production of continuous basalt fibers. ACS Omega 6:21465–21471
Chen M, Shi J, Taskinen P, Jokilaakso A (2020) Phase equilibria of the CaO-SiO2-TiO2-Al2O3-MgO system in air at 1250–1400°C. Ceram Int 46:27702–27710
Wan X, Shi J, Qiu Y, Chen M, Li J, Liu C, Taskinen P, Jokilaakso A (2021) The effect of 15wt%Al2O3 addition on the equilibrium phase relations of CaO-SiO2-TiO2 system at 1400°C in air. Ceram Int 47:24802–24808
Jung IH, Van Ende MA (2020) Computational thermodynamic calculations: FactSage from CALPHAD thermodynamic database to virtual process simulation. Metall Mater Trans B 51:1851–1874
Bartram SF, Slepetys RA (1961) Compound formation and crystal structure in the system ZnO-TiO2. J Am Ceram Soc 44:493–499
DeVries RC, Roy R, Osborn EF (1955) Phase equilibria in the system CaO-TiO2-SiO2. J Am Ceram Soc 38:158–171
Funding
This study received financial support from Key Laboratory Open Project Fund of Metallurgical Emission Reduction and Resources Recycling (Anhui University of Technology), Ministry of Education (JKF22-02), National Natural Science Foundation of China (No. 52204310), China Postdoctoral Science Foundation (Grant number 2020TQ0059, 2020M570967). The Natural Science Foundation of Liaoning Province (2021-MS-083). The Fundamental Research Funds for the Central Universities (N2125010), Key Laboratory for Anisotropy and Texture of Materials, Ministry of Education, National Natural Science Foundation of China (52074004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest and the authors alone are responsible for the content and writing of the article.
Additional information
The contributing editor for this article was Veena Sahajwalla.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, N., Qiu, Y., Hu, Y. et al. Equilibrium Phase Relationships of CaO-SiO2-TiO2 System with 5 wt% Cr2O3 Addition for Titania-Bearing Slag Recycling. J. Sustain. Metall. 9, 1303–1314 (2023). https://doi.org/10.1007/s40831-023-00723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00723-z