Abstract
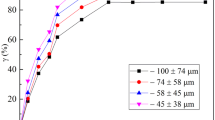
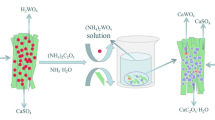
Recently, our research group proposed a new approach for converting calcium tungstate to tungsten trioxide via sodium bisulfate roasting, which has the potential to overcome the excessive water consumption associated with ion exchange technology and reduce excessive wastewater production. To investigate the mechanism of scheelite decomposition based on sodium bisulfate roasting, thermogravimetric analysis-differential scanning calorimetry (TG-DSC), X-ray diffraction (XRD) and scanning electron microscopy-energy dispersive spectrometry (SEM‒EDS) were used to systematically study the reaction course, phase transitions and kinetics of scheelite decomposition by sodium bisulfate roasting. The results of TG-DSC showed that when the temperature was T ≥ 50 °C, the crystal water was removed from NaHSO4·H2O, and NaHSO4·H2O was converted to NaHSO4. As the temperature increased to 120 °C ≤ T ≤ 270 °C, H+ was released from molten NaHSO4 and reacted with CaWO4 to generate H2WO4, which further decomposed into WO3 under the action of heat. When sodium bisulfate reacted with scheelite, a higher temperature and sodium bisulfate dosage were necessary to ensure that the molten sodium bisulfate destroyed the mineral lattice and fully contacted the CaWO4 in the mineral. XRD characterization results indicated that the primary products of scheelite after roasting with sodium bisulfate were WO3, CaSO4 and Na2SO4. A kinetic study showed that the sodium bisulfate roasting-induced scheelite decomposition reaction was in line with a kinetic equation for a first-order reaction, and the activation energy was 51.8 kJ·mol−1, suggesting that the reaction was controlled by a chemical reaction.
Graphical Abstract













Similar content being viewed by others
References
US Geological Survey (2020) Mineral commodity summaries 2020. US Geological Survey, Reston. https://doi.org/10.3133/mcs2020
Tang P-Z, Wang S-C, Wang J (2021) Global Tungsten Consumption history analysis and Demand Forecast. Nat Resour Econ China 34(1):55–59. https://doi.org/10.19676/j.cnki.1672-6995.000557
Ministry of Land and Resources of the People's Republic of China (2019) China mineral resources report. https://www.mnr.gov.cn/sj/sjfw/kc_19263/zgkczybg/201910/t20191022_2473040.html
Tang L, Wang P, Graedel TE, Pauliuk S, Chen WQ (2020) Refining the understanding of China’s tungsten dominance with dynamic material cycle analysis. Resour Conserv Recycl 158:104829. https://doi.org/10.1016/j.resconrec.2020.104829
Zhequan Yu (2020) Current situation analysis and suggestions of Tungsten industry in China. Land Resour Inf 10:55–60. https://doi.org/10.3969/j.issn.1674-3709.2020.10.010
Zhao Z, Sun F, Yang J, Fang Qi, Jiang W, Liu X, Chen X, Li J (2019) Development status and prospect of tungsten resources, technology and industry in China. Trans Nonferrous Met Soc China 29(9):1902–1916. https://doi.org/10.19476/j.ysxb.1004.0609.2019.09.07
Wan L, Xu G, Yan Y, Nie H, Zhao L, Xiao X (2009) Development and technological progress of tungsten smelting process in China. China Tungsten Ind 24(5):63-66+92
Wan L, Yang S, Zhao L, Li H (2012) The technology progress and development of APT green smelting in China. Adv Mater Res 30(6):550-553+682-686. https://doi.org/10.4028/www.scientific.net/amr.550-553.682
He L, Liu X, Zhao Z, Liang Y (2012) Study on alkali decomposition of tungsten minerals. China Tungsten Ind 27(02):22–27
Sheng C, Dai L, Qing X, Li J, Zhou Z, Ming L (2019) Analysis of the decomposition theory and process of scheelite. Value Eng 26:148–150
Ministry of Environmental Protection of the People's Republic of China (2016) National catalogue of hazardous waste. https://www.mee.gov.cn/gkml/sthjbgw/qt/201606/t20160621_354849.html
Ouyang T, Li M, Appel E, Tang Z, Zhu Z (2020) Magnetic response of Arsenic pollution in a slag covered soil profile close to an abandoned tungsten mine, southern China. Sci Rep 10:4357. https://doi.org/10.1038/s41598-020-61411-6
Zhao Z, Cao C, Li H (2008) Sodium carbonate decomposition of scheelite thermodynamics analysis. Chin J Nonferrous Met 17(2):356–360. https://doi.org/10.3321/j.issn:1004-0609.2008.02.028
Zhang J, Ju C, Chen L, Wang Y, Nie X, Zhao S (2021) Optimization of leaching process of sodium scheelite carbonate. China Tungsten Ind 36(4):60–64
He L, Zhao Z, Yang J (2017) A new generation of green tungsten metallurgy process: scheelite, sulfur and phosphoric acid Co-decomposition Technology. China Tungsten Ind 32(3):49–53. https://doi.org/10.3969/j.issn.1009-0622.2017.03.009
Guo F, Chen X, Zhao Z, He L, Yang K, Yang Z (2018) Sulfur phosphorus mixed acid decomposition associated rare earth in the process of scheelite behavior. Chin J Nonferrous Met 28(2):389–396. https://doi.org/10.19476/j.ysxb.1004.0609.2018.02.21
Wan L, Deng D, Zhao L, Li H, Xu G, Liang Y (2013) Research direction and technical progress of green tungsten metallurgy. Nonferrous Met Sci Eng 4(5):15–18
Wan L, Nie H, Tan D (2016) Technology of green smelting and high value development and utilization of scheelite resources. Sci Technol Inf 14(11):165–166
Sun J, Tongchun Wu (2015) Optimization control of flotation based on foam and grade analysis. Mod Min 9(9):73–80
Liang Y, Shao L, Li Y, Wang H, Liu P (2018) Sodium metasilicate nonahydrate digestion of scheelite in low-temperature roast. Chinese J Rare Met 42(6):668–672. https://doi.org/10.13373/j.cnki.cjrm
He B, Xu L, Lei X, Hu H, Liang Y, Liu D (2022) Study on decomposition of scheelite with low consumption of sodium phosphate. Rare Met Cem Carbide 50(2):1–7
He B, Liang Y, Fan Z, Xu L, Liu D, Xu G (2022) Dissolution behavior of sodium phosphate in a Na3PO4-Na2WO4-NaOH solution system. Minerals 12:732–743
Liang Y, Shao L, Li Y, Liang X, Wang H, Liu P (2018) Sodium silicate scheelite roasted at low temperature decomposition technology research. Chin J Rare Met 42(6):668–672. https://doi.org/10.13373/j.cnki.cjrm.XY17040028
Yang J, Che W, Hou K (2021) Application of electric heating rotary kiln in roasting and flotation of scheelite concentrate. China Tungsten Ind 36(1):20–27. https://doi.org/10.3969/j.issn.1009-0622.2021.01.004
Zherui H (1994) Application of ion exchange method intungsten metallurgy. China Tungsten Ind 5:1–4
Li H, Tang Z, He L, Chen X (2014) Application and progress of ion exchange technology in tungsten metallurgy. China Tungsten Ind 29(5):34–39. https://doi.org/10.3969/j.issn.1009-0622.2014.05.11
Shi M, Tang Z, Chen X (2015) Status and development of wastewater treatment in modern tungsten smelting process. Rare Met Cem Carbide 2:1–5
Xiong Y, Huanrong Yu, Chang Hu, Liu D (2014) High concentration ammonia nitrogen wastewater treatment out of tungsten and cobalt smelting. China Tungsten Ind 29(2):4. https://doi.org/10.3969/j.issn.1009-0622.2014.02.15
Yin Z, Zhao X (1994) Solubility of calcium sulfate in of hydrochloric acid and sodium chloride. Oilfield Chem 11(4):345–347
Liang Y (2015) Purification and preparation of special-shaped calcium carbonate and calcium sulfate whiskers from industrial by-product gypsum. Master's thesis, Southwest University of Science and Technology, Mianyang
Xu L, He B, Xu G, Lei X, Hu H, Liang Y, Liu D (2023) Study of equilibrium dissolution of WO3-CaSO4 in HCl-Na2SO4 solution. Chin J Eng 46(6):883–889. https://doi.org/10.13374/j.issn2095-9389.2022.03.25.001
Vries K, Gellings PJ (1969) The thermal decomposition of potassium and sodium-pyrosulfate. J Inorg Nucl Chem 31(5):1307–1313. https://doi.org/10.1016/0022-1902(69)80241-1
Ford A, Tariq S (1981) Molten potassium pyrosulfate: the reactions of alkali metal hydroxides, hydrogen carbonates and carbonates. Aust J Chem 34(3):647. https://doi.org/10.1071/ch9810647
Liu Y (1993) Manual of physicochemical properties and important reaction equations of inorganic substances. Chengdu University of Science and Technology Press, Chengdu, pp 300–301
Guo C, Zou J, Ma S, Yang J, Wang K (2019) Alumina extraction from coal fly ash via low-temperature potassium bisulfate calcination. Minerals 9(10):585. https://doi.org/10.3390/min9100585
Padilla R, Sohn HY (1985) Sintering kinetics and alumina yield in lime-soda sinter process for alumina from coal wastes. Metall Trans B 16:385–395. https://doi.org/10.1007/BF02679731
Rodriguez M, Quiroga O, Ruiz MDC (2007) Kinetic study of ferrocolumbite dissolution in hydrofluoric acid medium. Hydrometallurgy 85:87–94. https://doi.org/10.1016/j.hydromet.2006.07.005
Kahruman C, Yusufoglu I (2006) Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent. Hydrometallurgy 81:182–189. https://doi.org/10.1016/j.hydromet.2005.12.003
Acknowledgements
We are sincerely grateful the Qingjiang Program for Young Talents of Jiangxi University of Science and Technology (JXUSTQJBJ2017004) and the Natural Science Foundation of Jiangxi Province (20202ACBL204002) for financial supports of our research.
Funding
This study was funded by the Qingjiang Program for Young Talents of Jiangxi University of Science and Technology (JXUSTQJBJ2017004) and the Natural Science Foundation of Jiangxi Province (20202ACBL204002).
Author information
Authors and Affiliations
Contributions
LX: Writing—original manuscript and editing. YL: Methodology, funding acquisition and conceptualization. GX: Supervision. CH: Writing—review. BH: Writing—Data calculation, XZ: Validation. DL: Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was M. Akbar Rhamdhani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Liang, Y., Xu, G. et al. Decomposition Mechanism of Scheelite based on Sodium Bisulfate Roasting. J. Sustain. Metall. 9, 1061–1074 (2023). https://doi.org/10.1007/s40831-023-00709-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00709-x