Abstract
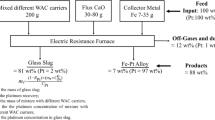
Spent automotive catalysts consist of platinum group metals (PGMs) in their leaching residue (LR) after wet extraction and are the leading secondary resource of PGMs. Herein, PGMs were captured from the LR of spent automotive catalysts by NiSO4 smelting, followed by treatment with sulfuric acid to enrich PGMs from the trap. The effects of the trap, fluxing agent, and slagging agent dosage on the recovery of PGMs were systematically investigated to determine the optimum smelting conditions. The capture efficiency was 90.13, 91.02, and 90.54% for Pt, Pd, and Rh, respectively, under the following conditions: NiSO4/LR mass ratio of 3.6, SiO2/LR mass ratio of 2.0, CaCO3/LR mass ratio of 1.0, Na2CO3/LR mass ratio of 0.3, Na2B4O7⋅10H2O/LR mass ratio of 0.3, CaF2/LR mass ratio of 0.3, and C/LR mass ratio of 0.4 at 1400 °C for 2 h. During the acid leaching enrichment process, the content of PGMs in the enrichment slag was 8.66 times compared with that of the raw materials under the conditions of 14.24 mol/L H2SO4, liquid–solid ratio of 5:1, 400 rpm, and temperature of 90 °C for 2 h. The method of combined smelting and wet extraction provides a referential value for the enrichment of PGMs from secondary resources.
Graphical Abstract













Similar content being viewed by others
Abbreviations
- PGMs:
-
Platinum group metals
- LR:
-
Leaching residue
References
Bernardis FL, Grant RA, Sherrington DC (2005) A review of methods of separation of the platinum-group metals through their chloro-complexes. React Funct Polym 65:205–217. https://doi.org/10.1016/j.reactfunctpolym.2005.05.011
Yakoumis I, Panou M, Moschovi AM, Panias D (2021) Recovery of platinum group metals from spent automotive catalysts: a review. Clean Eng Technol 3:100112. https://doi.org/10.1016/j.clet.2021.100112
Lanaridi O, Sahoo AR, Limbeck A, Naghdi S, Bica-Schrder K (2020) Toward the recovery of platinum group metals from a spent automotive catalyst with supported ionic liquid phases. ACS Sustain Chem Eng 9:375–386. https://doi.org/10.1021/acssuschemeng.0c07384
Willner J, Kaduková J, Fornalczyk A, Mrážiková A, Marcinčáková R, Velgosová O (2015) Possibilities of metals extracton from spent metallic automotive catalytic converters by using biometallurgical method. Arch Metall Mater 60:1877–1880. https://doi.org/10.1515/amm-2015-0320
Fajar A, Hanada T, Goto M (2021) Recovery of platinum group metals from a spent automotive catalyst using polymer inclusion membranes containing an ionic liquid carrier. J Membr Sci Res 629:119296. https://doi.org/10.1016/j.memsci.2021.119296
Ferella F (2019) A review on management and recycling of spent selective catalytic reduction catalysts. J Clean Prod 246:118990. https://doi.org/10.1016/j.jclepro.2019.118990
Wang WQ, Zhang L, Han Y, Zhang YC, Liu XG, Xu SM (2019) Cleaner recycling of spent Ni–Mo/γ-Al2O3 catalyst based on mineral phase reconstruction. J Clean Prod 232:266–273. https://doi.org/10.1016/j.jclepro.2019.05.375
Wei X, Liu CW, Cao HB, Ning PG, Jin W, Yang ZB, Wang HJ, Sun Z (2019) Understanding the features of PGMs in spent ternary automobile catalysts for development of cleaner recovery technology. J Clean Prod 239:118031. https://doi.org/10.1016/j.jclepro.2019.118031
Zhang LG, Song QM, Liu Y, Xu ZM (2019) Novel approach for recovery of palladium in spent catalyst from automobile by a capture technology of eutectic copper. J Clean Prod 239:118093. https://doi.org/10.1016/j.jclepro.2019.118093
Dong H, Zhao J, Chen J, Wu Y, Li B (2015) Recovery of platinum group metals from spent catalysts: a review. Int J Miner Process 145:108–113. https://doi.org/10.1016/j.minpro.2015.06.009
Peng ZW, Li ZZ, Lin XL, Tang HM, Ye L, Ma YT, Rao MJ, Zhang YB, Li GH, Jiang T (2017) Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM 69:1553–1562. https://doi.org/10.1007/s11837-017-2450-3
Benson M, Bennett CR, Harry JE, Patel KM (2000) The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour Conserv Recycl 31:1–7. https://doi.org/10.1016/S0921-3449(00)00062-8
Juvonen R, Lakomaa T, Soikkeli L (2002) Determination of gold and the platinum group elements in geological samples by ICP-MS after nickel sulphide fire assay: difficulties encountered with different types of geological samples. Talanta 58:595–603. https://doi.org/10.1016/S0039-9140(02)00330-2
Hoffmann JE (1988) Recovery of platinum-group metals from gabbroic rocks metals from auto catalysts. JOM 40:40–44. https://doi.org/10.1007/BF03258173
Kolliopoulos G, Balomenos E, Giannopoulou I, Yakoumis I, Panias D (2014) Behavior of platinum group metals during their pyrometallurgical recovery from spent automotive catalysts. Open Acess Library 1:e736. https://doi.org/10.4236/oalib.1100736
Ivanović SZ, Trujuć VK, Gorgievski MD, Mišić LD, Božić DS (2011) Removal of platinum group metals from the spent automobile catalyst by the pyrometallurgical process. 15th international research/expert conference on trends in the development of machinery and associated technology, Prague, Czech Republic, 12–18 September 2011. pp 701–704
Dong H, Zhao J, Chen J, Fan X, Fu G, Yang H (2014) Recovery of platinum group metal secondary resource by iron trapping method based on solid state. Chin J Nonferrous Metals 24:2692–2697
He X, Li Y, Wu X, Zhao Y, Wang H, Liu W (2016) Study on the process of enrichment platinum group metals by plasma melting technology. Precious Metals 37:1–5. https://doi.org/10.3969/j.issn.1004-0676.2016.01.001
Liu C, Sun S, Zhu X, Tu G (2020) Metals smelting-collection method for recycling of platinum group metals from waste catalysts: a mini review. Waste Manag Res 39:43. https://doi.org/10.1177/0734242X20969795
Jones RT, Geldenhuys IJ (2011) The pros and cons of reductive matte smelting for PGMs. Miner Eng 24:495–498. https://doi.org/10.1016/j.mineng.2011.03.007
Schalkwyk R, Eksteen JJ, Akdogan G (2013) Leaching of Ni–Cu–Fe–S converter matte at varying iron endpoints; mineralogical changes and behaviour of Ir, Rh and Ru. Hydrometallurgy 136:36–45. https://doi.org/10.1016/j.hydromet.2013.02.008
You G, Fang W, Li Q, Ma Y, Yang H (2016) Study on enrichment method of platinum, palladium and rhodium in spent auto-catalysts. Metall Anal 36:7–11. https://doi.org/10.13228/j.boyuan.issn1000-7571.009680
Kim BS, Lee JC, Jeong J, Yang DH, Shin D, Lee KI (2013) A novel process for extracting precious metals from spent mobile phone PCBs and automobile catalysts. Mater Trans 54:1045–1048. https://doi.org/10.2320/matertrans.M2013051
Willner J, Fornalczyk A, Cebulski J, Janiszewski K (2014) Preliminary studies on simultaneous recovery of precious metals from different waste materials by pyrometallurgical method. Arch Metall Mater 59:801–804. https://doi.org/10.2478/amm-2014-0136
Kayanuma Y, Okabe TH, Maeda M (2004) Metal vapor treatment for enhancing the dissolution of platinum group metals from automotive catalyst scrap. Metall Mater Trans B 35:817–824. https://doi.org/10.1007/s11663-004-0075-8
Li S, Yan J, Meng H, Xing F (2022) Recovery and enrichment of platinum from spent Al2O3 carrier catalysts by matte smelting-acid leaching process. Adv Mater Sci Eng 2022:1–9. https://doi.org/10.1155/2022/8473452
Acknowledgements
The authors greatly acknowledge the financial support from the Reserve Talent Program for Young and Middle-aged Academic and Technical Leaders in Yunnan Province (201905C160070) and the National Key Research and Development Program—“Science and Technology for Economy 2020” Key Special Project (SQ2020YFF0404678).
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Fan, X., He, Y. et al. Enrichment of Platinum Group Metals from Spent Automotive Catalyst Leaching Residue by a Combined Smelting and Wet Extraction Method. J. Sustain. Metall. 9, 1180–1189 (2023). https://doi.org/10.1007/s40831-023-00702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00702-4