Abstract
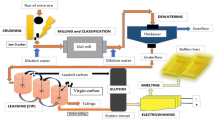
Recovery of zinc and safe disposal of electric arc furnace dust (EAFD) is an urgent problem. Ammonia–ammonium bicarbonate solutions can selectively leach zinc from EAFD. However, the method for zinc precipitation from leachate concerns quality of zinc products and efficiency of the process. Recently, precipitation of zinc by using gaseous CO2 as precipitant attracted wide attention by industry. To fully develop its industrial application, the optimal conditions of zinc precipitation from Zn-ammonia solutions by adding gaseous CO2 were investigated. The variation of CO2 absorption efficiency, alkalinity, and total ammonia during zinc recovery process was studied. The properties of zinc-precipitating products were systematically analyzed. Under the optimized zinc precipitating conditions, 80% of zinc was recovered as basic carbonate of hydrozincite (Ζn5(CO3)2(OH)6). During the process, CO2 absorption efficiency dropped to 32.4%, alkalinity and total ammonia decreased 2 mol/L and 0.6 mol/L, respectively. High-grade zinc-precipitating products of Zn5(CO3)2(OH)6 were obtained, which could thermally decompose to ZnO in temperature region 200–290 °C. The zinc precipitate particles were 2D plate-structured flakes and further agglomerated into 3D irregular spherical particles and columnar structures. The results will provide valuable parameters for industrial treatment of EAFD.
Graphical Abstract












Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Binnemans K, Jones PT, Fernandez AM, Torres VM (2020) Hydrometallurgical processes for the recovery of metals from steel industry by-products: a critical review. J Sustain Metall 6:505–540. https://doi.org/10.1007/s40831-020-00306-2
Li H-X, Wang Y, Cang D-Q (2010) Zinc leaching from electric arc furnace dust in alkaline medium. J Cent South Univ T 17:967–971. https://doi.org/10.1007/s11771-010-0585-2
Monteiro RCC, Lopes AK, Pinto J, Castro F (2015) Brass smelting dust as a source of ZnO in the production of targets used in magnetron sputtering thin film deposition. Wastes—solutions, treatments, opportunities 3rd International Conference
European waste catalogue and harzardous waste list (2002) Environmental protection agency: Ireland.
De Buzin PJWK, Heck NC, Vilela ACF (2017) EAF dust: an overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J Mater Res Technol 6:194–202. https://doi.org/10.1016/j.jmrt.2016.10.002
Kirankumar T, Roy GG (2022) A review on processing of electric arc furnace dust (EAFD) by pyro-metallurgical processes. Trans Indian Inst Met 75:1101–1112
Agency USEP (2021) Resource Conservation and Recovery Act (RCRA) regulations. In: Part 268-land disposal restrictions, pp 171–304.
Agency USEP (1991) Land disposal restrictions for electric arc furnace dust (K061)
Palimąka P, Pietrzyk S, Stępień M, Ciećko K, Nejman I (2018) Zinc recovery from steelmaking dust by hydrometallurgical methods. Metals-Basel. https://doi.org/10.3390/met8070547.
Nakamura T, Takasu T, Itou H (2008) Basic consideration on EAF dust treatment using hydrometallurgical processes. Res Prog 55:144–148
Quijorna N, De Pedro M, Romero M, Andres A (2014) Characterisation of the sintering behaviour of Waelz slag from electric arc furnace (EAF) dust recycling for use in the clay ceramics industry. J Environ Manag 132:278–286. https://doi.org/10.1016/j.jenvman.2013.11.012
Halli P, Hamuyuni J, Leikola M, Lundstrom M (2018) Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Miner Eng 124:1–9. https://doi.org/10.1016/j.mineng.2018.05.011
Jeanfrenay (1985) Leaching of oxidized zinc ores in various media. Hydrometallurgy 15:243–253. https://doi.org/10.1016/0304-386X(85)90057-X.
Liptai BDP (2020) Briančin J, Havlik T (2020) Hydrometallurgical recycling of rlectric arc furnace dust-application possibilities of ZnO product for the manufacture of varistors in the electrotechnical industry. Waste Biomass Valor 11:4419–4428. https://doi.org/10.1007/s12649-019-00722-w
Rodriguez NR, Gijsemans L, Busse J, Roosen J, Onal MAR, Torres VM, Fernandez AM, Jones PT, Binnemans K (2020) Selective removal of zinc from BOF sludge by leaching with mixtures of ammonia and ammonium carbonate. J Sustain Metall 6:680–690. https://doi.org/10.1007/s40831-020-00305-3
Ruiz O, Clemente C, Alonso M, Alguacil FJ (2007) Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J Hazard Mater 141:33–36. https://doi.org/10.1016/j.jhazmat.2006.06.079
Xanthopoulos P, Agatzini-Leonardou S, Oustadakis P, Tsakiridis PE (2017) Zinc recovery from purified electric arc furnace dust leach liquors by chemical precipitation. J Environ Chem Eng 5:3550–3559. https://doi.org/10.1016/j.jece.2017.07.023
Török TVTI (2013) Experimental investigation of zinc precipitation from EAF dust leaching solutions. Mater Sci Eng 38(1):61–71.
Wylock CE, Antuñano N, Arias PL, Haut B (2014) Analysis of the simultaneous gas–liquid CO2 absorption and liquid–gas NH3 desorption in a hydrometallurgical waelz oxides purification process. Int J Chem React Eng 12:549–562. https://doi.org/10.1515/ijcre-2014-0034
Palimąka P, Pietrzyk S, Stępień M, Ciećko K, Nejman I (2018) Zinc recovery from steelmaking dust by hydrometallurgical methods. Metals. https://doi.org/10.3390/met8070547
Harvey TG (2006) The hydrometallurgical extraction of zinc by ammonium carbonate: a review of the schnabel process. Min Proc Ext Met Rev 27:231–279. https://doi.org/10.1080/08827500600815271
Jiang F-YMT, Gao W, Zeng Y, Su H-H, Li Q, Xu B, Yang Y-B, Zhong Q (2021) Leaching behavior of zinc from crude zinc oxide dust in ammonia leaching. J Central South Univ 28(2021):2711–2723. https://doi.org/10.1007/s11771-021-4803-x
Herrero D, Arias PL, Cambra JF, Antunano N (2010) Hydrometallurgical processes development for zinc oxide production from waelz oxide. Waste Biomass Valori 1:329–337. https://doi.org/10.1007/s12649-010-9033-7
Chemical process design manual fourth edition, vol 1. Sinopec Group Shanghai Engineering Co. LTD, China (2009)
Liu TY, Sheng Y, Han LH, Liu Q (2017) Simulation of the bubble behaviors for gas-liquid dispersion in agitated vessel. J Chem Eng Jpn 50:4–14. https://doi.org/10.1252/jcej.16we023
Patwardhan AW, Joshi JB (1998) Design of stirred vessels with gas entrained from free liquid surface. Can J Chem Eng 76:339–364. https://doi.org/10.1002/cjce.5450760303
Zhang YQ, Pan X, Wang YH, Luo PC, Wu H (2018) Numerical and experimental investigation on surface air entrainment mechanisms of a novel long-short blades agitator. AlChE J 64:316–325. https://doi.org/10.1002/aic.15865
Yeh JT, Resnik KP, Rygle K, Pennline HW (2005) Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia. Fuel Process Technol 86:1533–1546. https://doi.org/10.1016/j.fuproc.2005.01.015
Vlek PLG, Stumpe JM (1978) Effects of solution chemistry and environmental conditions on ammonia volatilization loss from aqueous systems. Soil Sci Soc Am J 42:416–421. https://doi.org/10.2136/sssaj1978.03615995004200030008x
Wang JZ, Zongbo (2019) Determination of ammonium content in zinc electrolyte by formaldehyde method. Gansu Metall 41:99–100, 103.
Ma A, Zhang L, Peng J, Zheng X, Li S, Yang K, Chen W (2016) Extraction of zinc from blast furnace dust in ammonia leaching system. Green Process Synth 5:23–30. https://doi.org/10.1515/gps-2015-0051
Barzagli F, Mani F, Peruzzini M (2011) From greenhouse gas to feedstock: formation of ammonium carbamate from CO2 and NH3 in organic solvents and its catalytic conversion into urea under mild conditions. Green Chem 13:1267–1274. https://doi.org/10.1039/c0gc00674b
Hu J, Chen Q, Hu H, Ma Q, Yin Z, Hu F (2013) Extraction of zinc(II) from ammoniacal solution into hydrophobic ionic liquids. J Chem Technol Biot 88:644–650. https://doi.org/10.1002/jctb.3880
Hu J, Chen Q, Yang X, Hu F, Hu H, Yin Z (2012) Extraction of zinc from ammoniacal solution with beta-diketone: a comparative study of solvents used. Sep Purif Technol 87:15–21. https://doi.org/10.1016/j.seppur.2011.11.006
Bavarella S, Brookes A, Moore A, Vale P, Di Profio G, Curcio E, Hart P, Pidou M, Mcadam EJ (2020) Chemically reactive membrane crystallisation reactor for CO2-NH3 absorption and ammonium bicarbonate crystallisation: kinetics of heterogeneous crystal growth. J Membr Sci. https://doi.org/10.1016/j.memsci.2019.117682
Han W, Yang K, Li D, Zhang Z, Ma J, Ni S, Yang X (2016) The fabrication and characterization of Zn5(CO3)2(OH)6 as a new anode material for lithium ion batteries. Mater Lett 164:148–151. https://doi.org/10.1016/j.matlet.2015.10.102
Li L, Conway W, Burns R, Maeder M, Puxty G, Clifford S, Yu H (2017) Investigation of metal ion additives on the suppression of ammonia loss and CO2 absorption kinetics of aqueous ammonia-based CO2 capture. Int J Greenh Gas Con 56:165–172. https://doi.org/10.1016/j.ijggc.2016.11.016
Budzianowski WM (2011) CO2 reactive absorption from flue gases into aqueous ammonia solutions: the NH3 slippage effect. Environ Prot Eng 37:5–19. https://doi.org/10.1007/s40831-020-00306-2
Yu H, Qi GJ, Wang SJ, Morgan S, Allport A, Cottrell A, Do T, Mcgregor J, Wardhaugh L, Feron P (2012) Results from trialling aqueous ammonia-based post-combustion capture in a pilot plant at munmorah power station: gas purity and solid precipitation in the stripper. Int J Greenh Gas Con 10:15–25. https://doi.org/10.1016/j.ijggc.2012.04.014
Antunano N, Cambra JF, Arias PL (2019) Hydrometallurgical processes for Waelz oxide valorization—an overview. Process Saf Environ Protect 129:308–320. https://doi.org/10.1016/j.psep.2019.06.028
Kondratyeva ES, Gubin AF, Kolesnikov VA (2016) Study of the extraction of zinc(II) ions from ammonia-sulfate solutions. Theor Found Chem Eng 50:83–86. https://doi.org/10.1134/s0040579516010103
Kubal M, Svab M, Cermak J, Borvsek A (2001) Mobilization of copper and zinc hydroxides through use of ammonia. Sep Sci Technol 36:3223–3238. https://doi.org/10.1081/ss-100107769
Li S, Chen W, Yin S, Ma A, Yang K, Xie F, Zhang L, Peng J (2015) Impacts of ultrasound on leaching recovery of zinc from low grade zinc oxide ore. Green Process Synth 4:323–328. https://doi.org/10.1515/gps-2015-0036
Boyd CE, Tucker CS, Somridhivej B (2016) Alkalinity and hardness: critical but elusive concepts in aquaculture. J World Aquacult Soc 47:6–41. https://doi.org/10.1111/jwas.12241
Li KK, Yu H, Tade M, Feron P (2014) Theoretical and experimental study of NH3 suppression by addition of Me(II) ions (Ni, Cu and Zn) in an ammonia-based CO2 capture process. Int J Greenh Gas Con 24:54–63. https://doi.org/10.1016/j.ijggc.2014.02.019
World Steel Association (2010) Steel industry by-products: project group report 2007–2009. World Steel Association, Brussels
Octavio Aguilar FT (2017) Pérez-Hernández R, Gómez R, Hernández-Gordillo A (2017) Novel preparation of ZnS from Zn5(CO3)2(OH)6 by the hydro- or solvothermal method for H2 production. Catal Today 287:91–98. https://doi.org/10.1016/j.cattod.2016.11.042
Wu J, Xue D (2009) Hierarchical Zn5(OH)6(CO3)2 structure assembled in an aqueous solution. Mod Phys Lett B 23:3911–3918. https://doi.org/10.1142/s0217984909021995
Paramo JA, Strzhemechny YM, Endo T, Orel ZC (2015) Correlation of defect-related optoelectronic properties in Zn5(OH)6(CO3)2/ZnO nanostructures with their Quasi-Fractal dimensionality. J Nanomater. https://doi.org/10.1155/2015/237985
Parveen MF, Umapathy S, Dhanalakshmi V, Anbarasan R (2010) Synthesis and characterization of nano-sized Zn(OH)2 and Zn(OH)2 /PVA nano-composite. Compos Interface 17:757–774. https://doi.org/10.1163/092764410x519417
Tzompantzi-Flores C, Cesar Castillo-Rodriguez J, Gomez R, Tzompantzi F, Perez-Hernandez R, De La Luz Tlapaya V, Eduardo Santolalla-Vargas C (2019) Synthesis and characterization of ZnZr composites for the photocatalytic degradation of phenolic molecules: addition effect of ZrO2 over hydrozincite Zn5(OH)6(CO3)2. J Chem Technol Biot 94:3428–3439. https://doi.org/10.1002/jctb.5928
Sinhamahapatra A, Giri AK, Pal P, Pahari SK, Bajaj HC, Panda AB (2012) A rapid and green synthetic approach for hierarchically assembled porous ZnO nanoflakes with enhanced catalytic activity. J Mater Chem 22:17227–17235. https://doi.org/10.1039/c2jm32998k
Tzompantzi F, Portillo-Vélez NS, Castillo-Rodríguez JC, Gómez R, Hernández RP, Santolalla-Vargas CE (2020) Preparation and characterization of the polycrystalline material Zn5(OH)6(CO3)2. Determination of the active species in oxide-reduction processes. Fuel Process Technol 281:1–10. https://doi.org/10.1016/j.fuel.2020.118471
Wahab R, Ansari SG, Kim YS, Dar MA, Shin H-S (2008) Synthesis and characterization of hydrozincite and its conversion into zinc oxide nanoparticles. J Alloys Compd 461:66–71. https://doi.org/10.1016/j.jallcom.2007.07.029
Acknowledgements
We sincerely thank the College of Environment and Ecology of Chongqing University and CISDI Thermal & Environmental Engineering Co., Ltd., for their research facilities, as well as the editors and anonymous reviewers for their valuable comments.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51209240), Technology Innovation and Application Demonstration of Chongqing Science and Technology Planning Project (Project No. cstc2018jscx-msybX0308), and Technology foresight and system innovation of Chongqing science and technology planning projects (Project No. cstc2021jsyj-zzysbAX0050).
Author information
Authors and Affiliations
Contributions
LG contributed to conceptualization, methodology, investigation, validation, and writing-original draft. QA contributed to conceptualization, methodology, validation, formal analysis, and supervision. ZL contributed to writing-original draft, formal analysis, visualization, and resources. SD contributed to formal analysis, writing-original draft, editing, and supervision. LL contributed to resources, investigation, formal analysis, validation, and supervision. LZ contributed to formal analysis and data curation. NJ contributed to visualization, writing-review, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, L., An, Q., Li, Z. et al. Zinc Precipitation from Ammonia Leaching Solutions of Electric Arc Furnace Dust by Carbon Dioxide. J. Sustain. Metall. 9, 896–907 (2023). https://doi.org/10.1007/s40831-023-00695-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00695-0