Abstract
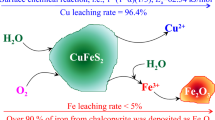
In this paper, a novel process leaching complex chalcopyrite without acid under oxygen pressure is proposed, and the leaching behavior and kinetic characteristics of copper in the complex chalcopyrite is studied. The results show that in the process of oxygen pressure leaching of complex chalcopyrite the oxidation dissolution of FeS2 occurs first, and then the sulfuric acid required by the reaction is generated, which destroys the embedded structure of various mineral phases in chalcopyrite and makes it dissolve into the leaching solution. The copper in the mineral reacts with sulfuric acid to form copper sulfate which enter into the leaching solution, and the iron in the mineral is mainly transformed to hematite and then remained in the leaching slag. Under the optimal leaching conditions (initial sulfuric acid concentration 0 g/L, reaction temperature 200 ℃, partial pressure of oxygen 1.2 MPa, mineral particle size − 48 + 38 µm), the leaching efficiency of copper reached 99.86% after 120 min reaction. The analysis of the kinetic process of oxygen pressure leaching of complex chalcolite based on the "shrinkage core model" showed that the leaching process of chalcopyrite is mainly controlled by chemical reaction. The apparent activation energy of the reaction is 50.646 kJ/mol. In the process of chemical reaction control, the parameters of partial pressure of oxygen and initial radius of mineral particles are 4.040 and − 0.773, respectively. The kinetic equation can be expressed as \(1 - \left( {1 - X} \right)^{\frac{1}{3}} = 1.123 \times 10^{4} \times {\text{P}}_{{{\text{O}}_{2} }}^{4.040} r_{0}^{ - 0.773} e^{{\frac{ - 6092}{T}}} t\).
Graphical Abstract















Similar content being viewed by others
References
Zhao HB, Wang J, Qin WQ et al (2015) Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms. Miner Eng 71:159–169
Yang BJ, Luo W, Wang XX et al (2020) The use of biochar for controlling acid mine drainage through the inhibition of chalcopyrite biodissolution. Sci Total Environ 737:139485
Yang BJ, Lin M, Fang JH et al (2020) Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans. Sci Total Environ 698:134175
Zhao HB, Wang J, Gan XW et al (2015) Cooperative bioleaching of chalcopyrite and silver bearing tailing by mixed moderately thermophilic culture: an emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis. Miner Eng 81:29–39
Wang J, Zhao HB, Qin WQ et al (2013) Investigation of interface reactions and electrochemical behaviors of chalcopyrite dissolution in different leaching mediums. Int J Electrochem Sci 12:12590–12599
Bai YL, Wang W, Xie F et al (2022) Effect of temperature, oxygen partial pressure and calcium lignosulphonate on chalcopyrite dissolution in sulfuric acid solution. Trans Nonferrous Met Soc China 32(5):1650–1663
Olubambi PA, Potgieter JH (2009) Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner Process Extr Metall Rev 30(4):327–345
Chen ML, Zhang L, Gu GH et al (2008) Effects of microorganisms on surface properties of chalcopyrite and bioleaching. Trans Nonferrous Met Soc China 6:1421–1426
Lane DJ, Cook NJ, Grano SR et al (2016) Selective leaching of penalty elements from copper concentrates: a review. Miner Eng 98:110–121
Petrovic SJ, Bogdanovic GD, Antonijevic MM (2018) Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans Nonferrous Met Soc China 28(7):1444–1455
Han B, Altansukh B, Haga K et al (2017) Leaching and kinetic study on pressure oxidation of chalcopyrite in H2SO4 solution and the effect of pyrite on chalcopyrite leaching. J Sustain Metall 3(3):528–542
Yevenes LV, Miki H, Nicol M (2010) The dissolution of chalcopyrite in chloride solutions: Part 2: effect of various parameters on the rate. Hydrometallurgy 103(1–4):80–85
Panda S, Akcil A, Pradhan N et al (2015) Current scenario of chalcopyrite bioleaching: a review on the recent advances to Its heap-leach technology. Biores Technol 196:694–706
Fowler TA, Crundwell FK (1998) Leaching of zinc sulfide by thiobacillus ferrooxidans: experiments with a controlled redox potential indicate no direct bacterial mechanism. Appl Environ Microbiol 64(10):3570–3575
Moyo T, Petersen J (2016) Study of the dissolution of chalcopyrite in solutions of different ammonium salts. J South Afr Inst Min Metall 116(6):509–516
Zhong S, Li YB (2019) An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl. J Market Res 8(4):3487–3494
Baba AA, Ghosh MK, Pradhan SR et al (2014) Characterization and kinetic study on ammonia leaching of complex copper ore. Trans Nonferrous Met Soc China 24(5):1587–1595
Okamoto H, Hiroyoshi N, Tsunekawa M (2009) Improved chalcopyrite leaching through optimization of redox potential. Can Metall Q 47(3):253–258
Ibanez T, Velasquez L (2013) The dissolution of chalcopyrite in chloride media. Rev Metal 49(2):131–144
Turan MD, Sari ZA, Nizamoglu H (2020) Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J Taiwan Inst Chem Eng 118:112–120
McDonald RG, Muir DM (2007) Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 86(3–4):191–205
Olvera OG, Rebolledo M, Asselin E (2016) Atmospheric ferric sulfate leaching of chalcopyrite: thermodynamics, kinetics and electrochemistry. Hydrometallurgy 165(1):148–158
Ruiz-Sanchez A, Lapidus GT (2022) Decomposition of organic additives in the oxidative chalcopyrite leaching with hydrogen peroxide. Miner Eng 187:107783
Bai YL, Wang W, Xie F et al (2022) Effect of temperature, oxygen partial pressure and calcium lignosulphonate on chalcopyrite dissolution in sulfuric acid solution. Trans Nonferrous Met Soc China 32:1650–1663
Zhang X, Zhang S (2022) Enhanced copper extraction in the chalcopyrite bioleaching system assisted by microbial fuel cells and catalyzed by silver-bearing ores. J Environ Chem Eng 10:108827
Hu JX, Zi FT, Tian GC (2021) Extraction of copper from chalcopyrite with potassium dichromate in 1-ethyl-3-methylimidazolium hydrogen sulfate ionic liquid aqueous solution. Miner Eng 172:107179
Bai YL, Wang W, Zhao SR et al (2022) Effect of mechanical activation on leaching behavior and mechanism of chalcopyrite. Miner Process Extr Metall Rev 43(4):440–452
Nie ZY, Zhang WW, Liu HC et al (2019) Bioleaching of chalcopyrite with different crystal phases by Acidianus manzaensis. Trans Nonferrous Met Soc China 29(3):617–624
Wu SF, Yang CR, Qin WQ et al (2015) Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C. Trans Nonferrous Met Soc China 25(12):4110–4118
Elsherief AE (2022) The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution. Miner Eng 15(4):215–223
Veglio F, Trifoni M, Pagnanelli F (2001) Shrinking core model with variable activation energy: a kinetic model of manganiferous ore leaching with sulphuric acid and lactose. Hydrometallurgy 60(2):167–179
Hiroyoshi N, Miki H, Hirajima T et al (2000) A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 57(1):31–38
Mahajan V, Misra M, Zhong K et al (2008) Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system. Miner Eng 20(7):670–674
Dickinson CF, Healb GR (1999) Solid–liquid diffusion controlled rate equations. Thermochim Acta 340(99):89–103
Shi GC, Liao YL, Su BW et al (2020) Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid. Sep Purif Technol 241:116699
Wang HH, Li GQ, Zhao D et al (2017) Dephosphorization of high phosphorus oolitic hematite by acid leaching and the leaching kinetics. Hydrometallurgy 171:61–68
Li L, Bian Y, Zhang X et al (2018) Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manage 71:362–371
Mu WN, Lu XY, Cui FH et al (2018) Transformation and leaching kinetics of silicon from low-grade nickel laterite ore by pre-roasting and alkaline leaching process. Trans Nonferrous Met Soc China 28(1):169–176
Li X, Han PW, Wang YL et al (2019) Silver dissolution in a novel leaching system: reaction kinetics study. Int J Miner Metall Mater 26(02):28–37
Zhou HM, Zheng SL, Zhang Y et al (2006) A kinetic study of the leaching of a low-grade niobium-tantalum ore by concentrated KOH solution. Hydrometallurgy 80(3):170–178
Aydogan S, Aras A, Canbazoglu M (2005) Dissolution kinetics of sphalerite in acidic ferric chloride leaching. Chem Eng J 114(1–3):67–72
Li M, Zhang XW, Liu ZG et al (2013) Kinetics of leaching fluoride from mixed rare earth concentrate with hydrochloric acid and aluminum chloride. Hydrometallurgy 140:71–76
He GX, Zhao ZW, Wang XB et al (2014) Leaching kinetics of scheelite in hydrochloric acid solution containing hydrogen peroxide as complexing agent. Hydrometallurgy 144:140–147
Dimitrios F, Rao K, George PD (1997) A kinetic study on the acid pressure leaching of pyrrhotite. Hydrometallurgy 47(1):1–18
Acknowledgements
The authors express their sincere appreciation to the National Natural Science Foundation of China for financial support (Project No. 21978122 and 21566017).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the content and agree to submit for consideration for publication in the journal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Zhongwei Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, G., Liao, Y., Xi, J. et al. Behavior and Kinetics of Copper During Oxygen Pressure Leaching of Complex Chalcopyrite Without Acid. J. Sustain. Metall. 9, 350–362 (2023). https://doi.org/10.1007/s40831-023-00658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00658-5