Abstract
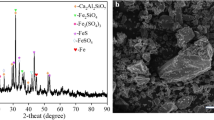
The presence of arsenic in base metal concentrates including copper, lead, and zinc creates problems such as low quality of final product and environmental issues. Therefore, additional processing steps for removing arsenic are required. Among several methods to reduce arsenic from these concentrates, alkaline leaching was selected as an efficient and simple method. In this research, sodium sulfide and sodium hydroxide mixtures were used for arsenic elimination from lead concentrate produced by Zarrin Madan Asia Company. To obtain optimum conditions, the effect of some parameters such as temperature, leaching time, liquid/solid ratio (L/S), sodium sulfide, and sodium hydroxide concentrations on arsenic removal was investigated. Based on characterization methods, mimetite was the main arsenic compound in lead concentrate; however, after alkaline sulfide leaching, the starting material was decomposed, and lead was mainly transformed to PbS. Accordingly, up to 98% of arsenic was removed under optimum selective leaching conditions (30 g/L of sulfide sodium, 5 g/L of sodium hydroxide, at 85 °C, 15 min, and L/S ratio = 7). To immobilize arsenic from alkaline leaching, the precipitation method was used by finding optimum conditions (at Fe/As = 3, 25 °C and 12 h). Therefore, the final product was suitable for further smelting processes without any environmental problems.
Graphical Abstract








Similar content being viewed by others
References
Liu B, Kim K-H, Kumar V, Kim S (2020) A review of functional sorbents for adsorptive removal of arsenic ions in aqueous systems. J Hazard Mater 388:121815. https://doi.org/10.1016/j.jhazmat.2019.121815
Yoshida H, Gao X, Koizumi S, Kim S-j, Ueda S, Miki T, Kitamura S-y (2017) Arsenic removal from contaminated water using the CaO–SiO2–FeO glassy phase in steelmaking slag. J Sust Metall 3(3):470–485. https://doi.org/10.1007/s40831-016-0108-y
Alka S, Shahir S, Ibrahim N, Ndejiko MJ, Vo D-VN, Manan FA (2021) Arsenic removal technologies and future trends: a mini review. J Clean Prod 278:123805. https://doi.org/10.1016/j.jclepro.2020.123805
Barakan S, Aghazadeh V (2019) Structural modification of nano bentonite by aluminum, iron pillarization and 3D growth of silica mesoporous framework for arsenic removal from gold mine wastewater. J Hazard Mater 378:120779. https://doi.org/10.1016/j.jhazmat.2019.120779
Obasi PN, Akudinobi BB (2020) Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl Water Sci 10(7):184. https://doi.org/10.1007/s13201-020-01233-z
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58(1):201–235. https://doi.org/10.1016/S0039-9140(02)00268-0
Vital M, Martínez DE, Babay P, Quiroga S, Clément A, Daval D (2019) Control of the mobilization of arsenic and other natural pollutants in groundwater by calcium carbonate concretions in the Pampean Aquifer, southeast of the Buenos Aires province, Argentina. Sci Total Environ 674:532–543. https://doi.org/10.1016/j.scitotenv.2019.04.151
Silin I, Hahn KM, Gürsel D, Kremer D, Gronen L, Stopić S, Friedrich B, Wotruba H (2020) Mineral processing and metallurgical treatment of lead vanadate ores. Minerals 10(2):197
Awe SA, Sandström Å (2010) Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner Eng 23(15):1227–1236. https://doi.org/10.1016/j.mineng.2010.08.018
Jarošíková A, Ettler V, Mihaljevič M, Drahota P, Culka A, Racek M (2018) Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust. J Environ Manag 209:71–80. https://doi.org/10.1016/j.jenvman.2017.12.044
Wikedzi A, Awe SA (2017) Selective extraction of antimony and arsenic from decopperization slime using experimental design. J Sust Metall 3 (2):362–374. https://doi.org/10.1007/s40831-016-0101-5
Spooren J, Binnemans K, Björkmalm J, Breemersch K, Dams Y, Folens K, González-Moya M, Horckmans L, Komnitsas K, Kurylak W, Lopez M, Mäkinen J, Onisei S, Oorts K, Peys A, Pietek G, Pontikes Y, Snellings R, Tripiana M, Varia J, Willquist K, Yurramendi L, Kinnunen P (2020) Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: technology development trends. Resour Conserv Recycl 160:104919. https://doi.org/10.1016/j.resconrec.2020.104919
Liu W, Li Z, Han J, Li W, Wang X, Wang N, Qin W (2019) Selective separation of arsenic from lead smelter flue dust by alkaline pressure oxidative leaching. Minerals 9(5):308. https://doi.org/10.3390/min9050308
Henao H, Paredes I, Diaz R, Ortiz J (2020) Pyrometallurgical removal of arsenic from dusts collected in electrostatic precipitators of copper. Part I—Dust from a flash smelting furnace. https://www.preprints.org/manuscript/202008.0377/v1
Shibayama A, Takasaki Y, William T, Yamatodani A, Higuchi Y, Sunagawa S, Ono E (2010) Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process. J Hazard Mater 181(1–3):1016–1023. https://doi.org/10.1016/j.jhazmat.2010.05.116
Leist M, Casey RJ, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76(1):125–138. https://doi.org/10.1016/S0304-3894(00)00188-6
Drahota P, Rohovec J, Filippi M, Mihaljevič M, Rychlovský P, Červený V, Pertold Z (2009) Mineralogical and geochemical controls of arsenic speciation and mobility under different redox conditions in soil, sediment and water at the Mokrsko-West gold deposit, Czech Republic. Sci Total Environ 407(10):3372–3384. https://doi.org/10.1016/j.scitotenv.2009.01.009
Tongamp W, Takasaki Y, Shibayama A (2009) Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 98(3–4):213–218. https://doi.org/10.1016/j.hydromet.2009.04.020
Brostow W, Gahutishvili M, Gigauri R, Hagg Lobland HE, Japaridze S, Lekishvili N (2010) Separation of natural trivalent oxides of arsenic and antimony. Chem Eng J 159(1–3):24–26. https://doi.org/10.1016/j.cej.2010.02.016
Yu G-l, Zhang Y, Zheng S-l, Zou X, Wang X-h, Zhang Y (2014) Extraction of arsenic from arsenic-containing cobalt and nickel slag and preparation of arsenic-bearing compounds. Trans Nonferr Met Soc China 24(6):1918–1927. https://doi.org/10.1016/S1003-6326(14)63272-6
Min X-b, Liao Y-p, Chai L-y, Yang Z-h, Xiong S, Liu L, Li Q-z (2015) Removal and stabilization of arsenic from anode slime by forming crystal scorodite. Trans Nonferr Met Soc China 25(4):1298–1306. https://doi.org/10.1016/S1003-6326(15)63728-1
Nazari AM, Radzinski R, Ghahreman A (2017) Review of arsenic metallurgy: treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 174:258–281. https://doi.org/10.1016/j.hydromet.2016.10.011
Guo X-y, Zhang L, Tian Q-h, Yu D-w, Shi J, Yi Y (2019) Selective removal of As from arsenic-bearing dust rich in Pb and Sb. Trans Nonferr Met Soc China 29(10):2213–2221. https://doi.org/10.1016/S1003-6326(19)65127-7
Shahnazi A, Firoozi S, Fatmehsari DH (2020) Selective leaching of arsenic from copper converter flue dust by Na2S and its stabilization with Fe2 (SO4)3. Trans Nonferr Met Soc China 30(6):1674–1686. https://doi.org/10.1016/S1003-6326(20)65329-8
Zhang X, Tian J, Han H, Sun W, Hu Y, Wang TYL, Yang Y, Cao X, Tang H (2020) Arsenic removal from arsenic-containing copper and cobalt slag using alkaline leaching technology and MgNH4AsO4 precipitation. Sep Purif Technol 238:116422. https://doi.org/10.1016/j.seppur.2019.116422
Long G, Peng Y, Bradshaw D (2012) A review of copper–arsenic mineral removal from copper concentrates. Miner Eng 36:179–186. https://doi.org/10.1016/j.mineng.2012.03.03235413
Xu Z-f, Li Q, Nie H-p (2010) Pressure leaching technique of smelter dust with high-copper and high-arsenic. Trans Nonferr Met Soc China 20(Supplement 1):s176–s181. https://doi.org/10.1016/S1003-6326(10)60035-0
Safarzadeh MS, Miller JD (2014) Reaction of enargite (Cu3AsS4) in hot concentrated sulfuric acid under an inert atmosphere. Part I: Enargite concentrate. Int J Miner Process 128:68–78. https://doi.org/10.1016/j.minpro.2014.02.007
Li Y, Liu Z, Li Q, Liu F, Liu Z (2016) Alkaline oxidative pressure leaching of arsenic and antimony bearing dusts. Hydrometallurgy 166:41–47. https://doi.org/10.1016/j.hydromet.2016.07.010
Welham NJ (2001) Mechanochemical processing of enargite (Cu3AsS4). Hydrometallurgy 62(3):165–173. https://doi.org/10.1016/S0304-386X(01)00195-5
Baláž P, Achimovičová M (2006) Selective leaching of antimony and arsenic from mechanically activated tetrahedrite, jamesonite and enargite. Int J Miner Process 81(1):44–50. https://doi.org/10.1016/j.minpro.2006.06.004
Twidwell L, Robins R, Hohn J (2005) The removal of arsenic from aqueous solution by coprecipitation with iron (III). Arsen Metall:3–24. https://www.researchgate.net/publication/235348967
Halder D, Lin J, Essilfie-Dughan J, Das S, Robertson J, Hendry MJ (2018) Implications of the iron (II/III)-arsenic ratio on the precipitation of iron-arsenic minerals from pH 2.5 to 10.5. Appl Geochem 98:367–376. https://doi.org/10.1016/j.apgeochem.2018.10.012
Acknowledgements
The authors acknowledge the support of Ayerma Industry and Mining Consulting Engineers Company with a Grant Number of 30/8582 for providing financial support to carry out this research.
Funding
This study was funded by Ayerma Industry and Mining Consulting Engineers Company (Grant Number of 30/8582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Christina Meskers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadizadeh, M., Barakan, S. & Aghazadeh, V. Arsenic Removal from Lead Concentrate-Containing Mimetite Mineral to Solve the Environmental Problem for Smelting Process. J. Sustain. Metall. 7, 1004–1012 (2021). https://doi.org/10.1007/s40831-021-00375-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00375-x