Abstract
Waste magnesia-carbon bricks (MCB) exhibit great potential for being reutilized as MgO additive for iron ore sintering due to their main composition of high activity magnesia and extra carbon content. In this paper, the combustion characteristics of the carbon component of waste MCB were first studied by thermogravimetric, derivative thermogravimetric and differential scanning calorimetry thermal analysis. The influence of waste MCB utilization as MgO additive on the mineralization of sinter raw materials was also systematically investigated. The main conclusions are shown as below: (1) Compared with anthracite, the carbon component of waste MCB has relatively worse flammability and combustibility, and its combustion heat per unit mass is less; (2) As the MgO content of sinter increases from 2.0 to 3.0 wt%, the advantage of using waste MCB, compared with dolomite, as MgO additive in the crystallinity degree of sinter gradually appears; (3) At the MgO content of 3.0 wt%, the sinter using the combination of waste MCB and dolomite as MgO additive has higher content of calcium ferrite, and the distributions of main mineral compositions and pores are more uniform than the sinter totally using dolomite or waste MCB as MgO additive.
Graphical Abstract

Similar content being viewed by others
Introduction
Australia has been accounting for the largest iron ore import share in China for many years. However, the iron ores imported from Australia generally contain high Al2O3 content, which would increase the Al2O3 content of blast furnace slag to a high level (> 15 wt%). Consequently, the slag viscosity is increased and the slag desulphurizing ability would be negatively affected [1]. Adjusting the MgO content of blast furnace slag to a proper level can effectively increase the slag fluidity and thus improve the slag desulphurizing ability [2,3,4]. The main MgO source of blast furnace slag is iron ore sinter [4]. However, it has been also known that over high MgO content has a negative effect on sinter strength and main sintering production indices [4,5,6,7,8]. Therefore, in practice, the MgO content of sinter is usually limited within 2.5 wt% [9, 10].
The MgO content of sinter mainly originates from the MgO additive of sinter raw materials. At present, dolomite has been widely used as MgO additive for sintering because of its lower price than other MgO-containing ores. However, there exists an issue that during the sintering process, the decomposition of dolomite would release CO2 and thus loosen the sinter structure. Furthermore, the decomposition of dolomite is an endothermic reaction, which indicates that during the sintering process the temperature around dolomite particles would fluctuate, leading to heat imbalance and further affecting the mineralization of high melting point oxides (e.g., MgO) with neighbor mineral components [11, 12]. Based on the above consideration, it is worth looking for an alternative MgO additive for sintering instead of dolomite so that the sinter quality can be still guaranteed at high MgO content.
Waste magnesia-carbon bricks (MCB) mainly refer to the refractory bricks unloaded from the inner side of the ladle or converter linings after longtime service. Since these bricks have been severely eroded, they cannot be recycled for making new MCB and hence become solid wastes [13]. However, the MgO content of waste MCB is generally higher than 65 wt% and exists as roasted magnesite with high purity and activity [14]. Furthermore, the carbon component of waste MCB (7–15 wt%) closely contacts with the MgO component. This structure helps improving the mineralization of MgO during sintering process because the carbon combustion can provide partial heat, which also saves the total fuel cost of sintering. This improved MgO mineralization would compensates the detrimental effect on sinter quality and sintering production indices when the MgO content of sinter exceeds a certain level (circa 2.5 wt%). Therefore, utilizing waste MCB as MgO additive could reasonably increase the MgO content of sinter and thus helps blast furnace slag gain better metallurgical properties. In general, applying waste MCB as new MgO additive for sintering not only realizes the efficient re-utilization of the solid waste containing valuable MgO source but also provides a new method of increasing the MgO content of sinter.
In this paper, the combustion characteristics of the carbon component of waste MCB, compared with anthracite, were first experimentally studied, aiming to discuss the difference of the combustion characteristics between these two fuel types during sintering process. The mineral compositions and microstructures of the sinters, respectively, using waste MCB, dolomite, and the combination of waste MCB and dolomite as MgO additives were compared, and the metallogenic mechanism in each sintering case was systematically analyzed. This research work is expected to lay a fundamental foundation for realizing the efficient re-utilization of waste MCB for iron ore sintering.
Methodology
Thermal Analysis Tests of Waste MCB Powder
To better understand the combustion characteristics of the carbon component of waste MCB powder, some key combustion characteristics indices were experimentally studied using thermal analysis and the experimental results were then compared with those of anthracite powder which was taken as a reference fuel type.
Table 1 shows the proximate analysis data, with main ash chemical compositions, of waste MCB and anthracite. Compared to anthracite, the carbon content of waste MCB is obviously lower but the ash content is significantly larger due to its main oxide compositions (especially MgO). In the preparation of combustion tests, the samples of waste MCB, anthracite, and magnesia were first dried at 110 °C for 12 h and then ground into the size range of − 0.074 mm. These ground samples were categorized into two groups (Group I: waste MCB; Group II: mixture of anthracite and magnesia). For Group II, the mass ratio of anthracite to magnesia was adjusted to guarantee that the mixture sample had the same carbon content with Group I. The combustion tests were carried out using a NETZSCH STA 449C simultaneous thermal analyzer. The test atmosphere was air and the air flow rate was 100 mL min−1. The mass of each group sample was 20 ± 0.2 mg for each test. During each combustion test, the temperature increased from 30 to 1200 °C with a rate of 5 °C min−1. After the test, the combustion characteristics, such as ignition temperature, maximum combustion rate, and combustion heat, of each group of sample were analyzed by the thermogravimetric (TG), derivative thermogravimetric (DTG), and differential scanning calorimetry (DSC) analyses. To guarantee the test accuracy, the combustion test for each group was repeated three times. The average data of the repeated test results were used for post-thermal analysis.
Analysis of the Mineral Composition and Microstructure of Sinter
The sinter raw materials used in the sintering experiment include iron ore fines, magnetite concentrate, slaked lime, dolomite, anthracite, and waste MCB. The binary basicity, TFe, and carbon content of the sintering mixture were set as 2.2, 56 wt%, and 3.5 wt%, respectively. The main chemical compositions of iron ores and fluxes are shown in Table 2. The MgO additives used in the sintering tests were grouped into (A) waste MCB, (B) dolomite and (C) the combination of waste MCB and dolomite. For Group C, MgO was equally provided by waste MCB and dolomite. For each group, there were three sintering tests, in which the MgO content varied from 2.0 to 3.0 wt% with an interval of 0.5 wt%. According to the experimental plan, totally nine sintering tests were designed and the details of each test are shown in Table 3.
The sinter raw materials of each test were first ground into the size range of − 0.074 mm and then fully mixed. About 2 g of the mixture was compressed under 10 MPa into a cylindrical sample (Φ8 mm × 5 mm). The sample was then sintered using an infrared ray mirror reflection rapid heating sintering device, as shown in Fig. 1. The test lasted about 30 min. The sintering atmosphere was air. The furnace temperature first increased from 30 to 1280 °C with a rate of 200 °C min−1 and then stayed constant at the peak for 2 min. Afterwards, the sinter product naturally cooled down to the room temperature. The detailed temperature variation during the test is shown in Fig. 2.
The main mineral compositions of the sintered sample of each test were analyzed using the X-ray diffraction (XRD) method. The mineral composition distribution and the microstructure of the sintered sample were observed using an OLYMPUS GX53 metallographic microscope. Moreover, the area proportions of mineral compositions and porosity of sintered sample were estimated using the image processing software—ImageJ. The mineral compositions and pores of sintered sample were first detected by threshold segmentation and marked with different colors. Then the pixels with the same color in the image were counted. The fraction of mineral composition i could be calculated by Ni/Nm, where Ni and Nm are the pixel numbers of mineral composition i and of all mineral compositions, respectively. The porosity could be calculated by Np/Nt, where Np and Nt are the pixel numbers of pores and of the whole image, respectively.
Results and Discussion
Combustion Characteristics of Waste MCB
The TG-DTG curves obtained from the average data of repeated combustion test results of waste MCB and the mixture of anthracite and magnesia (hereafter called A + M mixture) are compared in Fig. 3. It can be seen that the average ignition temperature, Ti, at which the sample weight started to decrease significantly (marked by the blue dash line), of the waste MCB powder was 695 °C, as shown in Fig. 3a. In contrast, Ti of A + M mixture was 460 °C, as shown in Fig. 3b. Furthermore, during the combustion period, the TG curve of the waste MCB powder showed a relatively less steep trend compared with the TG curve of A + M mixture. The average maximal combustion rate, Rmax, defined as the maximum of the absolute value of DTG, of the waste MCB powder was 0.703 (% min−1). In contrast, Rmax of the A + M mixture was 1.009 (% min−1). The values of Tmax, defined as the temperature corresponding to Rmax, of the waste MCB powder and the A + M mixture were 838 °C and 523 °C, respectively.
The flammability and combustibility of the test samples were evaluated by the indices of Ca and Cb. The definition of these two indices is shown as below [15, 16]:
Ca mainly reflects the reaction ability of carbon fuels at the early stage of combustion. A higher Ca indicates that the fuel sample could start combustion at a lower temperature and the combustion process is more violent. Cb evaluates the fuel combustibility more comprehensively by taking Tmax into account. A higher Cb indicates that the peak of fuel combustion rate occurs earlier during the combustion period, which could provide a better heating condition. Therefore, during the sintering process, the fuel sample with higher Ca and Cb normally ignites more easily and shows stronger combustion reaction, hence benefiting the fast heat release at the early stage of combustion.
Figure 4 shows the TG-DSC curves obtained from the average data of repeated combustion test results of the waste MCB powder and the A + M mixture. According to the DSC curves of the test samples, the average exothermic peak of waste MCB powder was 2.08 mW mg−1, which was lower than the value of A + M mixture (4.12 mW mg−1). Furthermore, the exothermic peak of waste MCB powder was wider than that of A + M mixture. This indicates that, compared to A + M mixture, the waste MCB powder experienced a relatively slower exothermic process and the burnout time was longer. The overall comparison of the combustion characteristics between waste MCB powder and A + M mixture was summarized as shown in Table 4.
In general, the comparison results shown in Table 4 indicated that compared with anthracite, the carbon component of waste MCB has relatively worse flammability and combustibility, and its combustion heat per unit mass is less. This could be explained by the physical properties of these two types of carbon fuels. The carbon component of waste MCB powder is graphite which has a regular layered molecule structure. This structure is dense, stable, and has a very high thermal conductivity [17]. Therefore, although the waste MCB powder has been ground to < 0.1 mm, its combustion condition is still difficult [18] and the combustion effectiveness is relatively low. In contrast, the main compositions of anthracite powder are the large organic polymers which contain multiple carbocyclic rings [19]. This structure is complex, irregular, and has a relatively lower thermal conductivity than graphite. As a result, its combustion condition is better than graphite and the combustion heat per unit mass is also higher than graphite. Considering the above analysis, it is concluded that using the carbon component of waste MCB as the partial solid fuel for sintering is possible but may not provide the equivalent heat per unit mass as anthracite does. However, since the carbon component of the waste MCB powder closely contacts with high activity magnesia, its combustion heat can be used by MgO more efficiently and hence improving the local mineralization of MgO.
Crystallinity of Mineral Compositions of Sinter
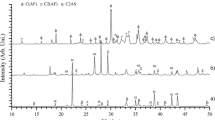
The XRD analysis was conducted to qualitatively investigate the mineral compositions of the sintered sample obtained from each sintering test. During the sintering process, the sinter raw materials experienced a series of complex solid-phase and liquid-phase reactions and ended up with the coexistence of several mineral compositions (e.g., hematite, magnetite, calcium ferrite, and silicate) in the sinter sample. The crystallinity degree, generally quantified by the diffraction peak of XRD pattern, is used to evaluate the assimilation process of the ore particles during the sintering process. Generally, the higher crystallinity degrees of the main mineral compositions indicate that the sinter chemical composition is simpler, which is beneficial to sinter strength because excessive minor mineral compositions remained may cause multiple stresses in the sinter structure during cooling process. Furthermore, a desirable amount of binding phase with proper strength (e.g., calcium ferrite) could be generated, which helps form a porous sinter structure with uniform small pores, hence improving both sinter strength and reducibility. According to the XRD analysis results, the influence of waste MCB and dolomite as MgO additive on the crystallinity degrees of main mineral compositions of sinter could be preliminarily understood. The XRD patterns of the three groups of sinter samples with different MgO contents are shown in Fig. 5.
The XRD patterns shown in Fig. 5 indicated that the main mineral compositions of the sinter samples were hematite, magnetite, calcium ferrite, and silicate. Through the comparison of the XRD patterns between Groups A, B, and C, it was observed that for the lowest MgO content cases (Row 1 in Fig. 5), when waste MCB was used as MgO additive (i.e., A1 and C1), the diffraction peaks of the main mineral compositions were relatively lower than the values of the sinter totally using dolomite as MgO additive (i.e., B1). This is mainly because that for A1 and C1, the graphite of waste MCB accounted for partial carbon content of the sintering mixture, and hence, the mass fraction of anthracite decreased to keep the total carbon content constant in each test. As analyzed in “Combustion Characteristics of Waste MCB” section, graphite has worse flammability and combustibility than anthracite. Therefore, compared with B1, the sinter raw materials of A1 and C1 reached the high temperature range relatively late and the high temperature period also lasted shortly during the sintering process. As a result, the crystallinity degrees of the main mineral compositions of A1 and C1 sinters were relatively lower than the values of B1 sinter.
With the MgO content increasing, the difference of the diffraction peaks of the main mineral compositions between Groups A, B, and C gradually decreased (Row 2 and Row 3 in Fig. 5). It is noticeable that at the MgO contents of 2.5 wt% and 3.0 wt%, the diffraction peaks of Group C were almost similar to the counterparts of Group B. This can be explained by the fact that as the MgO content increased from 2.0 to 3.0 wt%, the mass fraction of dolomite in Group B increased significantly (from 5.4 to 9.3 wt%). In contrast, since the MgO content of waste MCB is high, the mass fraction of dolomite in Group C only increased from 3.03 to 4.64 wt%, so the heat loss due to the decomposition of dolomite was limited to a large extent in Group C. As a result, Group C could have the similar crystallinity degree to Group B, even though Group B had a higher anthracite content.
In general, when utilizing waste MCB as MgO additive for the sintering case at high MgO content, the combination of waste MCB and dolomite could be considered as an alternative option. On the one hand, the addition of waste MCB could improve the overall MgO quality and limit the total amount of dolomite. On the other hand, the partial addition of waste MCB also limits the deficiency of graphite combustion compared to anthracite and hence guarantees the overall heat condition for the sinter raw materials. Therefore, the combination of waste MCB and dolomite is theoretically a reasonable choice which not only keeps the sinter quality at high MgO content but also reduces the cost of solid fuel.
Quantitative Analysis of Mineral Compositions of Sinter
The observation fields of the cross section of each cylindrical sinter sample were selected from five locations, including one central square and four random side squares (side length = 1.2 mm). The metallographic image of the central square of each sample and its corresponding threshold segmentation image illustrating main mineral compositions are shown in Fig. 6. In the images of threshold segmentation, the mineral compositions colored with dark blue, green, yellow, and red represent for hematite, magnetite, calcium ferrite, and silicate, respectively. The areas with light blue represent for the locations of pores. Based on the calculation results of the five segmentation images of each sample, the average fractions of the main mineral compositions and the average porosity of A1, B1, and C1 sinters are shown in Table 5.
Table 5 shows that compared with A1 and C1 sinters, B1 sinter contained higher calcium ferrite and silicate contents, but the hematite and magnetite contents were relatively lower. This is probably because that for B1 sinter, the combustion of higher content of anthracite provided a higher temperature environment for the generation of calcium ferrite and other mineralization processes. Since the MgO content was relatively low (2.0 wt%), for B1 sinter, the decomposition of dolomite consumed limited amount of heat and thus did not significantly affect the heat balance during the sintering process. Furthermore, Fig. 6 shows that the main mineral compositions of B1 sinter were relatively uniformly distributed, while the hematite of A1 sinter and the magnetite and calcium ferrite of C1 sinter were relatively densely distributed.
As shown in Table 5, the average porosities of A1 and B1 sinters were lower than the value of C1 sinter. Furthermore, Fig. 6 indicates that comparing with the porous structure of C1 sinter, A1 and B1 sinters had more large pores with thin walls and some large pores were even inter-connected. This is probably because that for B1 sinter, the higher contents of anthracite and dolomite resulted in a larger amount of CO2 generated during the sintering process. Moreover, since the combustion of higher content of anthracite leaded to a higher temperature environment, the fluidity of liquid phases consequently increased. Therefore, the generated gas was more easily encapsulated by the liquid phases which blocked the gaps between coarse grains, and the transfer of liquid phases between coarse grains promoted the coalescence of small-size pores. For A1 sinter, the combustion of lower content of anthracite resulted in a lower temperature environment, and consequently, limited amount of liquid phases with relatively lower fluidity was generated during the sintering process, which would influence the filling of liquid phases into the gaps between coarse grains and hence increased the sinter porosity.
The microstructures of A2, B2, and C2 sinters and the corresponding threshold segmentations of the main mineral compositions are shown in Fig. 7. The area fractions of the mineral compositions and porosity of A2, B2, and C2 sinters are shown in Table 6. As the MgO content increased from 2.0 to 2.5 wt%, for all three sinter samples, the magnetite and calcium ferrite contents increased and the hematite content decreased significantly. This change indicated that with increasing MgO content, the transition from magnetite to hematite was hindered due to the formation of magnesioferrite during the sintering process [20, 21]. On the other hand, MgO content could increase the fluidity of liquid phase, which benefited the diffusion of Ca2+ to hematite and thus increased the contact chance between iron oxide and CaO [22]. As a result, the hematite was increasingly consumed for the generation of calcium ferrite. Furthermore, the contents of the main mineral compositions, especially calcium ferrite and silicate, of these three sinter groups were similar. This illustrated that as MgO content increased to 2.5 wt%, using waste MCB as MgO additive (i.e., A2 and C2) began to show its advantage in the mineralization of sinter raw materials.
As the MgO content increased from 2.0 to 2.5 wt%, the porosity of A2 sinter almost kept constant at about 0.42 while the porosities of B2 and C2 sinters decreased by about 0.07 and 0.03, respectively. This change probably indicated that due to the decomposition of increasing amount of dolomite, the heat balances of B2 and C2 sinters were influenced to different degrees. In comparison to B2 sinter, the MCB component of C2 sinter could improve the mineralization of MgO and hence limited the heat fluctuation to some extent during sintering process, which also contributed to a uniform pore distribution. For Group A2 sinter, there was no decomposition of dolomite and hence the heat condition was relatively stable. Therefore, as shown in Fig. 7, although both A2 and B2 sinters contained large pores, A2 sinter had a more uniform pore distribution than B2 sinter.
The microstructures of A3, B3, and C3 sinters and the corresponding threshold segmentations of main mineral compositions are shown in Fig. 8. The area fractions of the main mineral compositions and porosity of A3, B3, and C3 sinters are shown in Table 7. With the MgO content further increasing from 2.5 to 3.0 wt%, the calcium ferrite content decreased and the magnetite content kept increasing. This indicated that over high content of MgO had an overall negative influence on the production of calcium ferrite through hindering a portion of magnetite getting oxidized into hematite, while it is also noticeable that, as the MgO content increased from 2.5 to 3.0 wt%, the calcium ferrite contents of A3, B3, and C3 sinters decreased by about 0.15, 0.12, and 0.10, respectively. This comparison illustrated that when using the combination of waste MCB and dolomite as MgO additive, the decrease in the content of calcium ferrite due to over high MgO content was the lowest. For high basicity sinter, calcium ferrite is the main binding phase that greatly contributes to the overall sinter strength. Therefore, the relatively higher content of calcium ferrite of C3 sinter indicated that as the MgO content increased to 3.0 wt%, the sinter strength could be still guaranteed through using the combination of waste MCB and dolomite as MgO additive. Furthermore, as shown in Fig. 8, the distributions of the main mineral compositions, especially calcium ferrite, of C3 sinter were more uniform than those of A3 and B3 sinters, which also helps improve the sinter strength via avoiding the serious stress concentrations at the boundaries between mineral phases.
As the MgO content increased from 2.5 to 3.0 wt%, the average porosity of A3 sinter slightly decreased by about 0.02 while more small-size pores existed with a relatively uniform distribution, as shown in Fig. 8. In comparison, the average porosity of B3 sinter increased by about 0.07 and there existed a portion of inter-connected large pores. This indicated that compared to dolomite, MCB could further improve the mineralization of MgO and stabilize the heat condition during the sintering process. For C3 sinter, although its overall porosity was relatively lower than A3 and B3 sinters, its porous structure showed more small-size pores with thick walls and the pore size distribution was more uniform. This structure is beneficial to improving both sinter strength and sinter reducibility. Based on the above analysis, the combination of waste MCB and dolomite is suggested as an alternative MgO addition method for sintering under a high MgO content condition. Further studies of the specific influence of different MgO addition methods on sinter strength and sinter reducibility have been conducted and will be published in near future.
Conclusions
To effectively re-utilize the waste magnesia-carbon bricks (MCB) in iron ore sintering, the combustion characteristics of waste MCB and the mineral compositions and microstructures of the sinters using waste MCB were experimentally studied. Based on the experimental results, the main conclusions are drawn as below:
-
(1)
By comparing the combustion characteristics between the carbon component of waste MCB and anthracite, it is concluded that the flammability and combustibility of the carbon component of waste MCB are worse than those of anthracite. The combustion of the carbon component of waste MCB provides less heat per unit mass than the value that the anthracite can provide.
-
(2)
At the MgO content of 2.0 wt%, the crystallinity degree of the sinter using dolomite as MgO additive is higher than the values of the sinters totally using or partially using waste MCB; as the MgO content increases to 3.0 wt%, the crystallinity degree of the sinter using the combination of waste MCB and dolomite as MgO additive almost equals to the value of the sinter totally using dolomite as MgO additive.
-
(3)
As MgO content increases from 2.0 to 3.0 wt%, using waste MCB as MgO additive gradually shows the advantages in improving the mineralization of MgO and stabilizing the heat condition during sintering process. At the MgO content of 3.0 wt%, the sinter using the combination of waste MCB and dolomite as MgO additive has higher content of calcium ferrite, and the distributions of main mineral compositions and pores are more uniform than the sinter totally using dolomite or waste MCB as MgO additive.
References
Du CK, Yang J, Zhao XZ et al (2013) Viscosity and desulfurization behavior of blast furnace slag with high Al2O3 content. J Iron Steel Res 25:19–22
Yang ST, Tan WD, Zhou M, Jiang T, Xue XX (2017) Effects of dolomite on mineral compositions and metallurgical properties of chromium-bearing vanadium–titanium magnetite sinter. Minerals 7:210–215
Wang XL (2014) Ferrous metallurgy (ironmaking part). Metallurgical Industry Press, Beijing
Yadav US, Pandey BD, Das BK, Jena DN (2002) Influence of magnesia on sintering characteristics of iron ore. Ironmak Steelmak 29:91–95
Kasai E, Sakano Y, Kawaguchi T, Nakamura T (2000) Influence of properties of fluxing materials on the flow of melt formed in the sintering process. ISIJ Int 40:857–862
Hsieh LH (2005) Effect of raw material composition on the sintering properties. ISIJ Int 45:551–559
Shen FM, Jiang X, Wu G et al (2006) Proper MgO addition in blast furnace operation. ISIJ Int 46:65–69
Cores A, Babich A, Muñiz M, Ferreira S, Mochon J (2010) The influence of different iron ores mixtures composition on the quality of sinter. ISIJ Int 50:1089–1098
Zhou CJ, Liu QM (2005) The practice for increasing sinter MgO content in Hangang. Sinter Pellet 30:37–40
Ke HB, Zhang YZ, Wang LL, Xing HW (2007) Effect of different contents of MgO on sinter quality. Henan Metall 15:8–10
Li Q, Huang ZC, Jiang T, Yang YB, Li GH (2006) Effect of dolomite and serpentine on sinter quality and microstructure. Iron Steel 41:10–14
Fan XH, Li WQ, Gan M et al (2012) Influence and mechanism of MgO on strength of high basicity sinter. J Cent South Univ (Sci Technol) 43:3325–3330
Zhang J, Bu JL, Wang LR, Wang RL (2013) Phase composition analysis of recycled MgO-C ladle lining materials. Adv Mater Res 750:2236–2239
Benavidez ER, Brandaleze E, Lagorio YS, Gladys A, Martinez T (2015) Thermal and mechanical properties of commercial MgO-C bricks. Rev Mater 20:571–575
Wang GW, Zhang JL, Zuo HB (2014) Study on the combustion characteristics and kinetics of pulverized coal with different particle size. Energy Metall Ind 33:49–53
Essenhigh RH, Misra MK, Shaw SW (1989) Ignition of coal particles: a review. Combust Flame 77:3–30
Deprez N, McLachlan DS (1988) The analysis of the electrical conductivity of graphite conductivity of graphite powders during compaction. J Phys D Appl Phys 21:101–106
Murakami K, Sugawara K, Kawaguchi T (2013) Analysis of combustion rate of various carbon materials for iron ore sintering process. ISIJ Int 53:1580–1587
Ye RQ, Xiang CS, Lin J et al (2013) Coal as an abundant source of graphene quantum dots. Nat Commun. https://doi.org/10.1038/ncomms3943
Dwarapudi S, Ghosh TK, Tathavadkar V et al (2012) Effect of MgO in the form of magnesite on the quality and microstructure of hematite pellets. Int J Miner Process 112:55–62
Sugiyama T, Shirouchi S, Tsuchi O et al (1982) High temperature reduction and softening properties of pellets with magnesite. Trans Iron Steel Inst Jpn 23:153–160
Deng T, Hu CQ (2014) Study on the distribution of MgO in the sinter. Henan Metall 22:8–11
Acknowledgements
The authors gratefully acknowledge the financial support for this work from the National Natural Science Foundation of China (No. 51874214), China Postdoctoral Science Foundation (No. 2020M672425), and Hubei Provincial Natural Science Foundation (No. 2020CFB133).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Yongxiang Yang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Yan, H., Zhang, W. et al. Fundamental Study on the Re-utilization of Waste Magnesia-Carbon Bricks as MgO Additive for Iron Ore Sintering. J. Sustain. Metall. 7, 597–609 (2021). https://doi.org/10.1007/s40831-021-00366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00366-y