Abstract
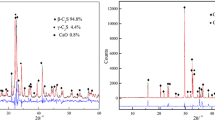
Steel slags generally swell when subjected to water or humidity, which prevents proper recycling in the cement or asphalt industries. The MgO and CaO phases in steel slags are responsible for this phenomenon, as both minerals easily absorb water to form their respective hydroxides. MgO is often present in steel slags in a solid solution with several oxides, constituting the so-called RO phase. This study investigates the hydration rate of an RO phase consisting of FeO and MgO called ferropericlase. The material was synthesized in a laboratory furnace by sintering a FeO–MgO powder mixture with varying initial FeO contents (approximately 10, 15, and 20 wt%). Thereafter, electron probe micro-analyzer (EPMA) and X-ray diffraction (XRD) spectroscopies were used to characterize the structure of the samples, which were mainly composed of ferropericlase and an exsolution of magnesioferrite. Also, Mössbauer spectra showed that the total ferrous iron proportion (Fe2+/ΣFe) of the sintered samples was in the range of 0.55–0.72. To measure the hydration behavior, the samples in powder form were cured in an autoclave at an H2O partial pressure of 2 atm. Thereafter, thermal gravimetric analysis (TGA) was performed to measure the amount of water absorbed during the autoclave curing from the mass drop associated with the dehydration of the hydroxide. The study found a linear correlation between the initial FeO content and the weight loss after TGA, with a reduction down to 6% in the sample with an initial FeO content of 20 wt% content compared to pure MgO.
Graphical Abstract










Similar content being viewed by others
References
Verhasselt A, Choquet F (1989) Steel slags as unbound aggregate in road construction: problems and recommendations. Unbound aggregates in roads. Elsevier, New York, pp 204–211
Mikhail SA, Turcotte AM (1995) Thermal behaviour of basic oxygen furnace waste slag. Thermochim Acta 263:87–94. https://doi.org/10.1016/0040-6031(94)02413-I
Motz H, Geiseler J (2001) Products of steel slags an opportunity to save natural resources. Waste Manage 21(3):285–293. https://doi.org/10.1016/S0956-053X(00)00102-1
Wang G, Wang Y, Gao Z (2010) Use of steel slag as a granular material: volume expansion prediction and usability criteria. J Hazard Mater 184(1–3):555–560. https://doi.org/10.1016/j.jhazmat.2010.08.071
Xie J (2012) Recycling of basic oxygen furnace slag in asphalt mixture: material characterization & moisture damage investigation. Construct Build Mater 36:467–474. https://doi.org/10.1016/j.conbuildmat.2012.06.023
Ortega-López V, Manso JM, Cuesta II, González JJ (2014) The long-term accelerated expansion of various ladle-furnace basic slags and their soil-stabilization applications. Construct Build Mater 68:455–464. https://doi.org/10.1016/j.conbuildmat.2014.07.023
Chen Z, Wu S, Wen J, Zhao M, Yi M, Wan J (2015) Utilization of gneiss coarse aggregate and steel slag fine aggregate in asphalt mixture. Construct Build Mater 93:911–918. https://doi.org/10.1016/j.conbuildmat.2015.05.070
Kambole C, Paige-Green P, Kupolati WK, Ndambuki JM, Adeboje AO (2017) Basic oxygen furnace slag for road pavements: a review of material characteristics and performance for effective utilisation in southern Africa. Construct Build Mater 148:618–631. https://doi.org/10.1016/j.conbuildmat.2017.05.036
Liu C, Guo M, Pandelaers L, Blanpain B, Huang S (2016) Stabilization of free lime in BOF slag by melting and solidification in air. Metall Mater Trans B 47(6):3237–3240. https://doi.org/10.1007/s11663-016-0809-4
Qian GR, Sun DD, Tay JH, Lai ZY (2002) Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. Brit Ceram Trans 101(4):159–164. https://doi.org/10.1179/096797802225003415
Otsuka K, Longo M, McCammon C, Karato S (2013) Ferric iron content of ferropericlase as a function of composition, oxygen fugacity, temperature and pressure: implications for redox conditions during diamond formation in the lower mantle. Earth Planet Sci Lett 365:7–16. https://doi.org/10.1016/j.epsl.2012.11.030
Narygina O et al (2009) High-pressure experimental and computational XANES studies of (Mg, Fe)(Si, Al)O3 perovskite and (Mg, Fe)O ferropericlase as in the Earth’s lower mantle. Phys Rev B 79(17):174115. https://doi.org/10.1103/PhysRevB.79.174115
Kung J, Li B, Weidner DJ, Zhang J, Liebermann RC (2002) Elasticity of (Mg0.83, Fe0.17)O ferropericlase at high pressure: ultrasonic measurements in conjunction with X-radiation techniques. Earth Planet Sci Lett 203(1):557–566. https://doi.org/10.1016/S0012-821X(02)00838-5
Holzapfel C, Rubie DC, Mackwell S, Frost DJ (2003) Effect of pressure on Fe–Mg interdiffusion in (FexMg1−x)O, ferropericlase. Phys Earth Planet Interiors 139(1–2):21–34. https://doi.org/10.1016/S0031-9201(03)00142-0
Otsuka K, Karato S (2015) The influence of ferric iron and hydrogen on Fe–Mg interdiffusion in ferropericlase ((Mg, Fe)O) in the lower mantle. Phys Chem Miner 42(4):261–273. https://doi.org/10.1007/s00269-014-0717-6
Ohta K, Yagi T, Hirose K, Ohishi Y (2017) Thermal conductivity of ferropericlase in the Earth’s lower mantle. Earth and Planetary Science Letters 465:29–37. https://doi.org/10.1016/j.epsl.2017.02.030
Ji X, Hou J, Liu Y, Liu J (2019) Effect of CaO-FeO-MnO system solid solution on the hydration activity of tri-component f-CaO in steel slag. Construct Build Mater 225:476–484. https://doi.org/10.1016/j.conbuildmat.2019.07.151
Xiao Y, Sun T, Zhao Y-H (2020) Experimental study on preparation of ferropericlase by oxalate coprecipitation. Minerals 10(2):179. https://doi.org/10.3390/min10020179
McCammon C, Peyronneau J, Poirier J-P (1998) Low ferric iron content of (Mg, Fe)O at high pressures and temperatures. Geophys Res Lett 25(10):1589–1592. https://doi.org/10.1029/98GL01178
Heidelbach F, Terry MP, Bystricky M, Holzapfel C, McCammon C (2009) A simultaneous deformation and diffusion experiment: quantifying the role of deformation in enhancing metamorphic reactions. Earth Planet Sci Lett 278(3–4):386–394. https://doi.org/10.1016/j.epsl.2008.12.026
Keppler H, Kantor I, Dubrovinsky LS (2007) Optical absorption spectra of ferropericlase to 84 GPa. Am Mineral 92(2–3):433–436. https://doi.org/10.2138/am.2007.2454
Tashiro M, Sukenaga S, Shibata H (2017) Control of crystallization behaviour of supercooled liquid composed of lithium disilicate on platinum substrate. Sci Rep 7:6078. https://doi.org/10.1038/s41598-017-06306-9
Arabi N, Jauberthie R (2012) Calcium silicate materials: substitution of hydrated lime by ground granulated blast furnace slag in autoclaving conditions. J Mater Civil Eng 24(9):1230–1236. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000480
Shi C, Hu S (2003) Cementitious properties of ladle slag fines under autoclave curing conditions. Cement Concr Res 33(11):1851–1856. https://doi.org/10.1016/S0008-8846(03)00211-4
Okamoto A, Futamura E, Kawamura K (1979) Hydration behavior of LD slag at autoclave test. Tetsu-to-Hagané 65(13):1878–1886. https://doi.org/10.2355/tetsutohagane1955.65.13_1878
Aydın S, Baradan B (2012) Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater Des 35:374–383. https://doi.org/10.1016/j.matdes.2011.10.005
Kourounis S, Tsivilis S, Tsakiridis PE, Papadimitriou GD, Tsibouki Z (2007) Properties and hydration of blended cements with steelmaking slag. Cement Concr Res 37(6):815–822. https://doi.org/10.1016/j.cemconres.2007.03.008
Tetens O (1930) Uber einige meteorologische Begriffe. Z. Geophys 6:297–309
Hou J, Chen Z, Liu J (2020) Hydration activity and expansibility model for the RO phase in steel slag. Metall Mater Trans B 51:1697–1704. https://doi.org/10.1007/s11663-020-01847-3
Longo M, McCammon C, Jacobsen S (2011) Microanalysis of the iron oxidation state in (Mg, Fe)O and application to the study of microscale processes. Contrib Mineral Petrol 162:1249–1257. https://doi.org/10.1007/s00410-011-0649-9
Turner RC, Hoffman I, Chen D (1963) Thermogravimetry of the dehydration of Mg(OH)2. Canad J Chem 41(2):243–251. https://doi.org/10.1139/v63-03
Acknowledgments
We would like to thank Prof. Ryo Inoue (Akita University) for the fruitful discussion and helpful advice on the methodology of autoclave curing of ferropericlase. We appreciate the technical support by Dr. Shingo Ishihara (Tohoku University) and Mr. Takashi Kamaya (Tohoku University) for the PSD and EPMA analysis of the samples, respectively. The Mössbauer measurements were performed at Nagoya Institute of technology under the Nanotechnology Platform Program of MEXT, Japan.
Funding
Funding was provided by JFE Steel Corporation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Colle, M., Sukenaga, S., Mibu, K. et al. Study of the Hydration Behavior of Synthetic Ferropericlase with Low Iron Oxide Concentrations to Prevent Swelling in Steel Slags. J. Sustain. Metall. 7, 547–558 (2021). https://doi.org/10.1007/s40831-021-00359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00359-x