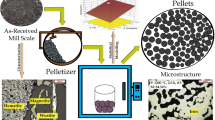
Abstract
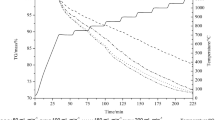
Mill scale is treated as a waste product by the steel industry, so it is essential to recycle and reuse this waste for recovery of metallic iron and its single oxide derivative. Most of the study in the field of mill scale reduction focuses on the static mill scale reduction. Optimizing the process in dynamic conditions becomes more difficult because of the complexity involved inside the process. In the present method, mill scale having particle size ranges from 106 to 53 µm was converted to single-phase oxide Fe2O3 by blowing pure oxygen at 1100 °C and then it was subjected to a reducing atmosphere of 50% H2 and 50% N2 (1:1) at 875 ± 5 °C at a varying reactor angle from 2° to 5° and different mass flow rates varying from 0.037 to 0.148 kg/min. A generalized experimental model (GEM) has been formulated for the degree of reduction of oxide, which encompasses twelve parameters consisting of reactor design and process parameters. A Buckingham theorem was used to obtain dimensionless parameter for the degree of reduction, and a logical correlation using the proposed model has been established.
Graphical Abstract









Similar content being viewed by others
References
Martín MI, López FA, Torralba JM (2013) Production of sponge iron powder by reduction of rolling mill scale. Ironmak Steelmak 39:155–162. https://doi.org/10.1179/1743281211Y.0000000078
Paswan D, Malathi M, Minj R, Bandhyopadhayay D (2015) Mill scale: a potential raw material for iron and steel making. Steelworld 21:54–56
Joshi C, Dhokey NB (2014) Study of kinetics of mill scale reduction: for PM applications. Trans Indian Inst Met 68:31–35. https://doi.org/10.1007/s12666-014-0425-4
Benchiheub O, Mechachti S, Serrai S, Khalifa MG (2010) Elaboration of iron powder from mill scale. J Mater Environ Sci 1(4):267–276
Pineau A, Kanari N, Gaballah I (2006) Kinetics of reduction of iron oxides by H2. Part I: Low-temperature reduction of hematite. Thermochim Acta 447:89–100. https://doi.org/10.1016/j.tca.2005.10.004
Gaballah NM, Zikry F, Khalifa MG, Farag B, El-Hussiny N, Shalabi MEH (2013) Production of iron from mill scale industrial waste via hydrogen. OJINM 03:23–28. https://doi.org/10.4236/ojinm.2013.33005
Sundaresan S (2000) Modeling the hydrodynamics of multiphase flow reactors: current status and challenges. AIChE J Am Inst Chem Eng 46(6):1102–1105. https://doi.org/10.1002/aic.690460602
Pannala S, Syamlal M, O'Brien TJ (2010) Computational gas-solids flows, and reacting systems: theory, methods, and practice. IGI Global, Hershey, PA
Themelis NJ (1972) Techniques of process analysis in extractive metallurgy. Metall Trans 3:2021–2029
Kritzinger HP, Kingsley TC (2015) Modelling and optimization of a rotary kiln direct reduction process. S Afr Inst Min Metall 115:419–424
Ghosh AA (2000) Secondary steel making principle and applications. CRC Press, Boca Raton, pp 299–317
Dhokey NB, Walunj MG, Chaudhary UC (2014) Influence of water pressure and apex angle on prediction of particle size for atomization of the copper powder. Adv Powder Technol 25:795–800
Gaurav GK, Khanam S (2017) Computational fluid dynamics analysis of sponge iron rotary kiln. Case Stud Therm Eng 9:14–27. https://doi.org/10.1016/j.csite.2016.11.001
Boateng AA (2013) Rotary kilns transport phenomena and transport processes. Elsevier, Oxford. ISBN: 978-0-7506-7877-3. https://doi.org/10.1017/CBO9781107415324.004
Indian patent filed (2019) Patent No. 201921048998, 28 Nov 2019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
The contributing editor for this article was Adam Clayton Powell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Derivation of Prediction of Dimensionless Degree of Reduction Model
Total no. variables (n) = 12.
Total no. fundamental dimensions (m) = 4.
No. of dimensionless (π) term = n − m = 12 − 4 = 8.
Repeating variables: (1) Characteristic length (L), (2) flow rate of gas (Qvg), (3) temperature of reduction (Tr), (4) density of H2 gas (ρg).
Finding out the expression for degree of reduction in terms of the above-mentioned parameters
Term can be calculated as follows:
For M, d = 0.
For L, a + 3b − 3d + 1 = 0, a = − 1.
For T, b = 0,
For θ, c = 0
Similarly,
Rights and permissions
About this article
Cite this article
Jikar, P.C., Dhokey, N.B. Influence of Process Parameters on Countercurrent Reactor Reduction of Oxidized Mill Scale Waste and Its Co-relationship with Mathematical Model. J. Sustain. Metall. 6, 622–630 (2020). https://doi.org/10.1007/s40831-020-00297-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00297-0