Abstract
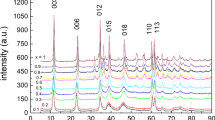
The recovery of Nd and Dy from a ternary Co–Nd–Dy system using layered crystalline zirconium phosphate (α-ZrP) was investigated. α-ZrP was synthesized by the refluxing method, and subsequently characterized by sodium hydroxide titration, Fourier transform-infrared spectrometry, thermogravimetry, scanning electron microscopy, and X-ray diffraction. The selectivity and ion exchange kinetics of the α-ZrP material were determined with regard to the individual elements. The influence of solution pH on the uptake was studied in ternary 1 and 2 mM equimolar solutions. The results showed that in acidic solution (pH 1–3), very little Co was taken up, while Nd and Dy uptakes were at reasonable levels (0.5–0.6 meq/g). The uptake isotherms of Nd, Dy, and Co were measured separately at pH 2.5 and 4.5 in ternary equimolar solution series. At pH 4.5, the loading capacities were about 0.2 meq/g for Co, 1.1 meq/g for Nd, and 1.5 meq/g for Dy. Dy was thus clearly preferred over Nd by α-ZrP. The loading of an α-ZrP column showed a similar preferred pattern. Nitric acid eluent removed Co, Nd, and Dy, but there was no separation of the metals in the eluate. A mixture of nitric and phosphoric acids, however, produced a strong separation. Co was very weakly retained in the column, and the ratio Dy/Nd in the eluent was in the range of 2–7. Thus, the α-ZrP material showed encouraging ion exchange properties for the separation and recycling of Nd and Dy from a ternary Co–Nd–Dy system. Much more work is needed, however, to develop a practical separation flowsheet.












Similar content being viewed by others
References
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22
Alonso E, Sherman AM, Wallington TJ, Everson MP, Field FR, Roth R, Kirchain RE (2012) Evaluating rare earth element availability: a case with revolutionary demand from clean technologies. Environ Sci Technol 46(6):3406–3414
Rademaker JH, Kleijn R, Yang Y (2013) Recycling as a strategy against rare earth element criticality: a systemic evaluation of the potential yield of NdFeB magnet recycling. Environ Sci Technol 47(18):10129–10136
Du X, Graedel T (2011) Global rare earth in-use stocks in NdFeB permanent magnets. J Ind Ecol 15(6):836–843
Reck BK, Graedel T (2012) Challenges in metal recycling. Science 337(6095):690–695
Walton A, Han Y, Mann V, Bevan A, Speight J, Harris I, Williams A (2012) The use of hydrogen to separate and recycle NdFeB magnets from electronic waste. In: Proceedings of the 22nd international workshop on rare earth magnets and their applications, Nagasaki, Japan
Sheridan R, Williams A, Harris I, Walton A (2014) Improved HDDR processing route for production of anisotropic powder from sintered NdFeB type magnets. J Radioanal Nucl Chem 350:114–118
Vander HT, Blanpain B, Van Gerven T, Binnemans K (2014) From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid. RSC Adv 4(109):64099–64111
Riaño S, Binnemans K (2015) Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem 17:2931–2942
Inamuddin LM, Luqman M (2012) Ion exchange technology I: theory and materials. Springer, Berlin, pp 978–994
Li N, Tompsett GA, Huber GW (2010) Renewable high-octane gasoline by aqueous-phase hydrodeoxygenation of C5 and C6 carbohydrates over Pt/zirconium phosphate catalysts. Chemsuschem 3(10):1154–1157
Mimura H, Lehto J, Harjula R (1997) Selective removal of cesium from simulated high-level liquid wastes by insoluble ferrocyanides. J Nucl Sci Technol 34(6):607–609
Gura V, Macy AS, Beizai M, Ezon C, Golper TA (2009) Technical breakthroughs in the wearable artificial kidney (WAK). Clin J Am Soc Nephrol 4(9):1441–1448
Alberti G, Casciola M (2001) Solid state protonic conductors, present main applications and future prospects. Solid State Ionics 145(1):3–16
Clearfield A, Stynes J (1964) The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J Inorg Nucl Chem 26(1):117–129
Clearfield A, Duax W, Garces J, Medina A (1972) On the mechanism of ion exchange in crystalline zirconium phosphates-IV potassium ion exchange of α-zirconium phosphate. J Inorg Nucl Chem 34(1):329–337
Kumar CV, Chaudhari A (2000) Proteins immobilized at the galleries of layered α-zirconium phosphate: structure and activity studies. J Am Chem Soc 122(5):830–837
Casciola M, Alberti G, Donnadio A, Pica M, Marmottini F, Bottino A, Piaggio P (2005) Gels of zirconium phosphate in organic solvents and their use for the preparation of polymeric nanocomposites. J Mater Chem 15(39):4262–4267
Vivani R, Alberti G, Costantino F, Nocchetti M (2008) New advances in zirconium phosphate and phosphonate chemistry: structural archetypes. Microporous Mesoporous Mater 107(1):58–70
Sun L, Boo WJ, Sue H-J, Clearfield A (2007) Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J Chem 31(1):39–43
Mosby BM, Díaz A, Bakhmutov V, Clearfield A (2013) Surface functionalization of zirconium phosphate nanoplatelets for the design of polymer fillers. ACS Appl Mater Interfaces 6(1):585–592
Trobajo C, Khainakov SA, Espina A, García JR (2000) On the synthesis of α-zirconium phosphate. Chem Mater 12(6):1787–1790
Horsley S, Nowell D, Stewart D (1974) The infrared and Raman spectra of α-zirconium phosphate. Spectrochim Acta A Mol Spectrosc 30(2):535–541
Rajeh A, Szirtes L (1995) Investigations of crystalline structure of gamma-zirconium phosphate. J Radioanal Nucl Chem 196(2):319–322
Helfferich F (1995) Ion exchange. Dover, New York
Clearfield A (1984) Inorganic ion exchangers with layered structures. Annu Rev Mater Sci 14(1):205–229
Harvie SJ, Nancollas GH (1970) Ion exchange properties of crystalline zirconium phosphate. J Inorg Nucl Chem 32(12):3923–3937
Clearfield A, Duax W, Medina A, Smith GD, Thomas J (1969) Mechanism of ion exchange in crystalline zirconium phosphates. I. Sodium ion exchange of. alpha.-zirconium phosphate. J Phys Chem 73(10):3424–3430
Clearfield A, Troup J (1970) Mechanism of ion exchange in crystalline zirconium phosphate. II. Lithium ion exchange of. alpha.-zirconium phosphate. J Phys Chem 74(2):314–317
Lee I-H, Kuan Y-C, Chern J-M (2007) Equilibrium and kinetics of heavy metal ion exchange. J Chin Inst Chem Eng 38(1):71–84
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276(1):47–52
Alkan M, Demirbaş Ö, Doğan M (2007) Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater 101(3):388–396
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Acknowledgments
The research leading to these results received funding from the European Community’s Seventh Framework Programme ([FP7/2007–2013]) under grant agreement no. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network, http://www.erean.eu). This publication reflects only the authors’ views, exempting the Community from any liability. The authors would like to thank Dr. Rikard Ylmen for his help in drawing the crystal structure.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was T. Hirato.
Rights and permissions
About this article
Cite this article
Xu, J., Wiikinkoski, E., Koivula, R. et al. HF-Free Synthesis of α-Zirconium Phosphate and Its Use as Ion Exchanger for Separation of Nd(III) and Dy(III) from a Ternary Co–Nd–Dy System. J. Sustain. Metall. 3, 646–658 (2017). https://doi.org/10.1007/s40831-017-0124-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-017-0124-6