Abstract
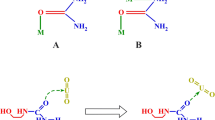
Eudialyte belongs to the group of cyclosilicate minerals and has a significant content of valuable heavy rare earth elements. In comparison to conventional ores like bastnaesite or monazite, the content of radioactive elements like thorium and uranium is quite low; thus, it is an ideal source for the sustainable extraction of rare earth elements (REE). In this way, a further processing or a disposal, direct or after using phase, of radioactive elements could be minimized. The cyclosilicate structure facilitates the easy decomposition of eudialyte by mineral acids. On one hand, this is a positive effect to lixiviate the REEs but leads to an excess liberation of silicon in the water-based system which involves the risk of silica gel formation. Within this research project, a hydrometallurgical pre-treatment is developed to decompose the eudialyte for the extraction of REEs and to stabilize the silicon in the residue during the following leaching step.











Similar content being viewed by others
References
Gambogi J (2016) Mineral Commodity Summaries 2016: Rare Earth, USGS National Minerals Information Center. http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/mcs-2016-raree.pdf. Accessed 11 Feb 2016
Lucas J (2014) Rare earths: science, technology, production and use. Elsevier, Amsterdam
Kingsnorth D (2015) The global rare earths industry today—plagued by illegal production in China. In: 11th international rare earths conference, Singapore
Zhang J (2016) Separation hydrometallurgy of rare earth elements. Springer
Yang XJ, Lin A, Li X et al (2013) China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ Dev 8:131–136. doi:10.1016/j.envdev.2013.03.006
Ministry of Environmental Protection (2011) Emission Standards of Pollutants from Rare Earth Industry. http://english.mep.gov.cn/standards_reports/standards/water_environment/Discharge_standard/201111/W020110210366768105784.pdf. Accessed 22 Mar 2016
Binnemans K, Jones PT (2015) Rare earths and the balance problem. J Sustain Metall 1(1):29–38. doi:10.1007/s40831-014-0005-1
Chakhmouradian AR, Wall F (2012) Rare earth elements: minerals, mines, magnets (and more). Elements 8(5):333–340. doi:10.2113/gselements.8.5.333
Goodenough KM, Schilling J, Jonsson E et al (2016) Europe’s rare earth element resource potential: an overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol Rev 72:838–856. doi:10.1016/j.oregeorev.2015.09.019
Giuseppetti G, Mazzi F, Tadini C (1971) The crystal structure of eudialyte. Tschermaks mineralogische und petrographische Mitteilungen 16:105–127
Johnsen O et al (2003) The nomenclature of eudialyte-groupe minerals. Can Miner 41:785–794
Ralph K, Ralph J Eudialyte. http://www.mindat.org/min-1420.html. Accessed 24 Nov 2015
Jordens A, Cheng YP, Waters KE (2013) A review of the beneficiation of rare earth element bearing minerals. Miner Eng 41:97–114. doi:10.1016/j.mineng.2012.10.017
Stoltz N, Friedrichs P (2015) Eudialyte as an alternative resource: mineralogy, occurrence and availability. Final report—S-FB rare earth green mining and separation. RWTH Aachen University, FZ Jülich, pp 11–15 (unpublished)
Schilling J, Wu F, McCammon C et al (2011) The compositional variability of eudialyte-group minerals. Miner Mag 75(1):87–115. doi:10.1180/minmag.2011.075.1.87
European Commission (2014) Report on critical raw materials for the EU: Report of the Ad hoc Working Group on defining critical raw materials. http://ec.europa.eu/enterprise/policies/raw-materials/files/docs/crm-report-on-critical-raw-materials_en.pdf. Accessed 22 May 2015
Merriman D. The European REE market and its place in the global industry. In: European Rare Earth Resources (ed) ERES 2014—1st International Conference on European Rare Earth Resources: Program and Book of Abstracts
Zaitsev V, Kogarko L Sources and perspectives of REE in the Lovozero massif (Kola Peninsula, Russia)
unbekannt 200. Stud., den 16. December 1819. In: unbekannt (ed) Göttingische gelehrte Anzeigen—Der dritte Band: Der königl. Gesellschaft der Wissenschaft, pp 1993–2000
Zakharov VI, Maiorov DV, Alishkin AR et al (2011) Causes of insufficient recovery of zirconium during acidic processing of lovozero eudialyte concentrate. Russ J Non-Ferr Met 52(5):423–428. doi:10.3103/S1067821211050129
Lebedev V, Shchur T, Maiorov D et al (2003) Specific features of acid decomposition of eudialyte and certain rare-metal concentrates from Kola Peninsula. Russ J Appl Chem 76(8):1191–1196
Lebedev V (2003) Sulfuric acid technology for processing of eudialyte concentrate. Russ J Appl Chem 76(10):1559–1563
Motov DL, Leshtaeva TG (1966) Chemical technology of rare-earth raw materials. Nauka, Moscow, pp 5–16 (in Russian)
Irwin AL (2013) Dubbo Zirconia Project: environmental impact statement. http://majorprojects.planning.nsw.gov.au/index.pl?action=view_job&job_id=5251%20. Accessed 14 Mar 2016
Verbaan N, Bradley K, Brown J et al (2015) A review of hydrometallurgical flowsheets considered in current REE projects. Symposium on critical and strategic materials proceedings. Ministry of Energy and Mines, British Columbia Geological Survey. http://www.empr.gov.bc.ca/Mining/Geoscience/PublicationsCatalogue/Papers/Documents/P2015-3/18%20Verbaan.pdf
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. Wiley, New York
Kokhanenko P, Brown K, Jermy M (2016) Silica aquasols of incipient instability: synthesis, growth kinetics and long term stability. Colloids Surf A 493:18–31. doi:10.1016/j.colsurfa.2015.10.026
Bergna HE (1994) The colloid chemistry of silica: developed from a symposium sponsored by the Division of Colloid and Surface Chemistry, at the 200th National Meeting of the American Chemical Society, Washington, DC, August 26–31, 1990. Washington, DC, pp 1–47
Forrester K, Krebs D, Voßenkaul D (2015) Informal meeting between GBM Minerals Engineering Consultants, Greenland Minerals and IME Process Metallurgy and Metal Recycling, RWTH Aachen University
Voßenkaul D (2016) Entwicklung eines Aufschlussverfahrens zur Gewinnung seltener Erdelemente aus Eudialyt (Development of a decomposition process of eudialyte to recover rare earth elements). Dissertation, Schriftenreihe des IME, RWTH, Shaker Verlag. http://www.shaker.de/de/content/catalogue/index.asp?lang=de&ID=6&category=280
Voßenkaul D, Friedrich B, Kruse S et al (2016) Method for opening a eudialyte mineral (DE102014218346A1)
Voßenkaul D, Friedrich B, Kruse S et al (2016) Method for opening a eudialyte mineral (EP2995692A1)
Pirrie D, Butcher AR, Power MR et al (2004) Rapid quantitative mineral and phase analysis using automated scanning electron microscopy (QemSCAN); potential applications in forensic geoscience. Geol Soc Lond Spec Publ 232(1):123–136. doi:10.1144/GSL.SP.2004.232.01.12
Krishnamurthy N, Gupta CK (2016) Extractive metallurgy of rare earths, 2nd edn. CRC Press, Taylor & Francis Group, Boca Raton
Gorrepati EA, Wongthahan P, Raha S et al (2010) Silica precipitation in acidic solutions: mechanism, pH effect, and salt effect. Langmuir 26(13):10467–10474. doi:10.1021/la904685x
Acknowledgments
This research is part of the Siemens AG collaborative research center S-FB “Green Mining and Separation” at the Aachen University. We thank the organizers of the 11th International Rare Earths Conference in Singapore for the possibility of an advanced presentation of these research findings.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was S. Kitamura.
Rights and permissions
About this article
Cite this article
Voßenkaul, D., Birich, A., Müller, N. et al. Hydrometallurgical Processing of Eudialyte Bearing Concentrates to Recover Rare Earth Elements Via Low-Temperature Dry Digestion to Prevent the Silica Gel Formation. J. Sustain. Metall. 3, 79–89 (2017). https://doi.org/10.1007/s40831-016-0084-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-016-0084-2