Abstract
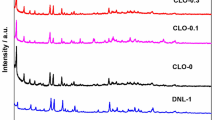
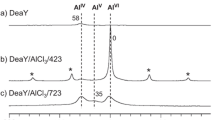
Zeolites with different framework structures (SSZ-13, ZSM-5, BEA) but similar Si/Al ratios (~ 12–15) and Pd loading (~ 1 wt%) were synthesized and evaluated as low-temperature passive NOx adsorbers (PNA). These materials exhibit high NOx adsorption efficiency with atomically dispersed Pd being the active adsorption site. Hydrothermal aging at 750 °C for 16 h in the presence of 10% water vapor in air resulted in the formation of PdO nanoparticles in all three samples as evidenced by high-energy XRD. Hydrothermal aging of the small-pore 1–3 wt% Pd/SSZ-13 (Si/Al = 6) materials, which contain ~ 100–90% atomically dispersed palladium ions, decreases its PNA performance only by ~ 10–20%, indicating agglomeration of only ~ 10–20% of atomically dispersed Pd into PdO. High-field solid state 27Al NMR studies on the fresh and aged samples reveal dealumination and significant changes in the distribution of Al (and thus, Brönsted acid) sites after hydrothermal aging. FTIR measurements with NO probe molecule and titration of Brönsted acid sites with nitrosyl (NO+) ions further corroborate the 27Al NMR data. Because framework aluminum atoms are the anchoring sites for atomically dispersed Pd ions, their elution from the framework causes the loss of active atomically dispersed Pd species. With the aid of HAADF-STEM imaging and synchrotron XRD studies, we further confirm and visualize the fate of these Pd species: they agglomerate into PdO nanoparticles on the external surface of zeolite. Consequently, these changes lead to the decrease in PNA performance of these materials after hydrothermal aging. The thus formed PdO agglomerates cannot be re-dispersed back to their ionic state due to the loss of framework Al T-sites and/or inherent stability of such large PdO particles. Our study demonstrates that, unlike in previous studies that found increased PNA performance upon HTA, high temperatures hydrothermal aging of PNA materials that contain atomically dispersed Pd initially results in a decrease in NOx storage efficiency due to the formation of PdO agglomerates. However, we also highlight the high hydrothermal stability of predominantly atomically dispersed 1–3 wt% Pd/SSZ-13 (Si/Al = 6), whose performance decreases only marginally after prolonged hydrothermal aging at 750 °C. This study shows that hydrothermally stable passive NOx materials can be prepared using small-pore SSZ-13 zeolite.



















Similar content being viewed by others
References
Khair, M.K., Majewski, W.A.: Diesel emissions and their control. SAE International, Warrendale (2006)
Forzatti, P., Nova, I., Tronconi, E.: New “enhanced NH3-SCR” reaction for NOx emission control. Ind. Eng. Chem. Res. 49(21), 10386–10391 (2010)
Beale, A., Gao, F., Lezcano-Gonzalez, I., Peden, C., Szanyi, J.: Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 44(20), 7371–7405 (2015)
Melville, J. E., Brisley, R. J., Keane, O., Phillips, P. R., Mountstevens, E. H.: US Patent US8105559B2 (2012)
Cole, J. A.: US Patent US5656244A (1997)
Murata, Y., Morita, T., Wada, K., Ohno, H.: NOx trap three-way catalyst (N-TWC) concept: TWC with NOx adsorption properties at low temperatures for cold-start emission control. SAE Int. J. Fuels Lubr. 8(2), 454–459 (2015)
Rajaram, R. R., Chen, H.-Y., Liu, D.: US Patent US20150158019A1 (2015)
Chen, H.-Y., Collier, J.E., Liu, D., Mantarosie, L., Duran-Martin, D., Novak, V., Rajaram, R., Thompsett, D.: Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 146, 1706–1711 (2016)
Ji, Y., Bai, S., Crocker, M.: Al2O3-based passive NOx adsorbers for low temperature applications. Appl. Catal. B Environ. 170–171, 283–292 (2015)
Jones, S., Ji, Y., Crocker, M.: CeO2-M2O3 passive NOx adsorbers for cold start applications, CLEERS Workshop (2016)
Zheng, Y., Kovarik, L., Engelhard, M.H., Wang, Y., Wang, Y., Gao, F., Szanyi, J.: Low-temperature Pd/zeolite passive NOx adsorbers: structure, performance, and adsorption chemistry. J. Phys. Chem. C. 121(29), 15793–15803 (2017)
Theis, J.R., Lambert, C.K.: Mechanistic assessment of low temperature NOx adsorbers for cold start NOx control on diesel engines. Catal. Today. (2017) In Press
Vu, A., Luo, J., Li, J., Epling, W.S.: Effects of CO on Pd/BEA passive NOx adsorbers. Catal. Lett. 147, 745–750 (2017)
Ryou, Y., Lee, J., Lee, H., Kim, C.H., Kim, D.H.: Effect of various activation conditions on the low temperature NO adsorption performance of Pd/SSZ-13 passive NOx adsorber. Catal. Today. (2017) In Press
Khivantsev, K., Gao, F., Kovarik, L., Wang, Y., Szanyi, J.: Molecular level understanding of how oxygen and carbon monoxide improve NOx storage in palladium/SSZ-13 passive NOx adsorbers: the role of NO+ and Pd(II)(CO)(NO) species. J. Phys. Chem. C. 122(20), 10820–10827 (2018)
Camblor, M.A., Corma, A., Valencia, S.: Synthesis in fluoride media and characterisation of aluminosilicate zeolite beta. J. Mater. Chem. 8(9), 2137–2145 (1998)
Prodinger, S., Shi, H., Wang, H., Derewinski, M.A., Lercher, J.A.: Impact of structural defects and hydronium ion concentration on the stability of zeolite BEA in aqueous phase. Appl. Catal. B Environ. 237, 996–1002 (2018)
Khivantsev, K., Jaegers, N.R., Kovarik, L., Hanson, J.C., Tao, F.F., Tang, Y., Zhang, X., Koleva, I.Z., Aleksandrov, H.A., Vayssilov, G.N., Wang, Y., Gao, F., Szanyi, J.: Achieving atomic dispersion of highly loaded transition metals in small-pore zeolite SSZ-13: high-capacity and high-efficiency low temperature CO and passive NOx adsorbers. Angew. Chem. Int. Ed. 57(51), 16672–16677 (2018)
Khivantsev, K., Jaegers, N.R., Kovarik, L., Prodinger, S., Derewinski, M.A., Wang, Y., Gao, F., Szanyi, J.: Palladium/beta zeolite passive NOx adsorbers (PNA): clarification of PNA chemistry and the effects of CO and zeolite crystallite size on PNA performance. App. Cat. A. 569, 141–148 (2019)
Khivantsev, K., Jaegers, N.R., Koleva, I.Z., Aleksandrov, H.A., Kovarik, L., Engelhard, M., Gao, F., Wang, Y., Vayssilov, G., Szanyi, J.: Stabilization of super electrophilic Pd+2 cations in small-pore SSZ-13 zeolite, Chemrxiv (2019) https://doi.org/10.26434/chemrxiv.7789454
Lee, J., Ryou, Y., Hwang, S., Kim, Y., Cho, S.-J., Lee, H., Kim, C., Kim, D.H.: Catal. Sci. Technol. 9, 163–173 (2019)
Kovarik, L., Washton, N.M., Kukkadapu, R., Devaraj, A., Wang, A., Wang, Y., Szanyi, J., Peden, C.H.F., Gao, F.: Transformation of active sites in Fe/SSZ-13 SCR catalysts during hydrothermal aging: a spectroscopic, microscopic, and kinetics study. ACS Catal. 7, 2458–2470 (2017)
Gao, F., Walter, E.D., Kollar, M., Wang, Y., Szanyi, J., Peden, C.H.F.: Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 319, 1–14 (2014)
Jaegers, N.R., Wan, C., Hu, M.Y., Vasiliu, M., Dixon, D.A., Walter, E., Wachs, I.E., Wang, Y., Hu, J.Z.: Investigation of silica-supported vanadium oxide catalysts by high-field 51V magic-angle spinning NMR. J. Phys. Chem. C. 121(11), 6246–6254 (2017)
Hu, J.Z., Zhang, X., Jaegers, N.R., Wan, C., Graham, T.R., Hu, M., Pearce, C.I., Felmy, A.R., Clark, S.B., Rosso, K.M.: Transitions in Al coordination during gibbsite crystallization using high-field 27Al and 23Na MAS NMR spectroscopy. J. Phys. Chem. C. 121(49), 27555–27562 (2017)
Qin, Z., Cychosz, K.A., Melinte, G., El Siblani, H., Gilson, J.-P., Thommes, M., Fernandez, C., Mintova, S., Ersen, O., Valtchev, V.: Opening the cages of faujasite-type zeolite. J. Am. Chem. Soc. 139(48), 17273–17276 (2017)
Kwak, J.H., Lee, J.H., Burton, S.D., Lipton, A.S., Peden, C.H.F., Szanyi, J.: A common intermediate for N2 formation in enzymes and zeolites: side-on Cu–nitrosyl complexes. Angew. Chem. Int. Ed. 52(38), 9985–9989 (2013)
Hadjiivanov, K.I.: Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 42, 71–144 (2000)
Hadjiivanov, K., Saussey, J., Freysz, J.L., Lavalley, J.C.: FT-IR study of NO + O2 co-adsorption on H-ZSM-5: re-assignment of the 2133 cm−1 band to NO+ species. Catal. Lett. 52(1/2), 103–108 (1998)
Szanyi, J., Paffett, M.T.: The adsorption of NO and reaction of NO with O2 on H-, NaH-, CuH-, and Cu-ZSM-5: an in situ FTIR investigation. J. Catal. 164(1), 232–245 (1996)
Khivantsev, K., Vityuk, A., Aleksandrov, H.A., Vayssilov, G.N., Blom, D., Alexeev, O.S., Amirdis, M.D., et al.: ACS Catal. 7, 5965–5982 (2017)
Khivantsev, K., Vityuk, A., Aleksandrov, H.A., Vayssilov, G.N., Alexeev, O.S., Amirdis, M.D.: Effect of Si/Al ratio and Rh precursor used on the synthesis of HY zeolite-supported rhodium carbonyl hydride complexes. J. Phys. Chem. C. 119(30), 17166–17181 (2015)
Epling, W.S., Campbell, L.E., Yezerets, A., Currier, N.W., Parks, J.E.: Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. Sci. Eng. 46, 163–245 (2004)
Choi, J.S., Partridge, W.P., Lance, M.J., Walker, L.R., Pihl, J.A., Toops, T.J., Finney, C.E.A., Daw, C.S.: Nature and spatial distribution of sulfur species in a sulfated barium-based commercial lean NOx trap catalyst. Catal. Today. 151, 354–361 (2010)
Lietti, L., Forzatti, P., Nova, I., Tronconi, E.J.: NOx storage reduction over Pt Ba/γ-Al2O3 catalyst. J. Catal. 204, 175–191 (2001)
Kim, D.H., Mudiyanselage, K., Szanyi, J., Zhu, H., Kwak, J.H., Peden, C.H.F.: Characteristics of Pt-K/MgAl2O4 lean NOx trap catalysts. Catal. Today. 184, 2–7 (2012)
Alikhani, M.E., Krim, L., Manceron, L.: Infrared spectra and structures of nickel and palladium dinitrosyl complexes isolated in solid argon. J. Phys. Chem. A. 105, 7817–7822 (2001)
Mintova, S., Gilson, J.P., Valtchev, V.: Advances in nanosized zeolites. Nanoscale. 5(15), 6693–6703 (2013)
Acknowledgements
We gratefully acknowledge the U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Program for the support of this work. Most of the research described in this paper was performed in the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory (PNNL). PNNL is operated for the US DOE by Battelle.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
KK, JSz, LK, NJ, FG, and YW filed for a patent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khivantsev, K., Jaegers, N.R., Kovarik, L. et al. Palladium/Zeolite Low Temperature Passive NOx Adsorbers (PNA): Structure-Adsorption Property Relationships for Hydrothermally Aged PNA Materials. Emiss. Control Sci. Technol. 6, 126–138 (2020). https://doi.org/10.1007/s40825-019-00139-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-019-00139-w