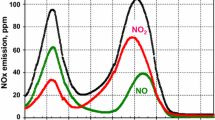
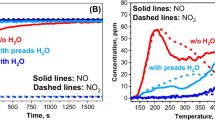
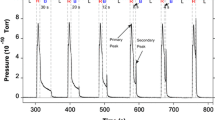
Abstract
Low temperature NOx adsorbers (LTNA) adsorb the NOx emissions from diesel engines during the cold start period and then thermally release the NOx when the downstream urea SCR system is operational. A degreened monolithic core sample of a LTNA with palladium on a ceria-zirconia washcoat (Pd/CZO) was evaluated for NOx storage performance under lean conditions using a reactor-based transient temperature test with and without different reductants [i.e., ethylene (C2H4), carbon monoxide (CO), hydrogen (H2), or a 3:1 CO:H2 mix]. Relative to tests without a reductant, all four reductants increased the amount of NOx stored on the LTNA. The NOx storage performance decreased gradually during consecutive tests. Lean methane (CH4) oxidation was used to probe the average oxidation state of the PdOx in the LTNA after the transient tests. The PdOx was reduced similarly on tests with NO alone and with NO and each reductant but significantly less with the reductant alone. NO alone reduced the PdOx by reacting with the PdOx to form NO2 between 200 and 210 °C. CH4 oxidation tests were also performed after the bed temperature exceeded 185 °C on transient tests with and without the reductants (i.e., after the NOx storage period). There was little PdOx reduction with NO alone or reductant alone, but there was significant and similar PdOx reduction with NO and each reductant. This supported a mechanism where NO and the reductant interacted to form a chemical intermediate on a PdOx site, reducing the PdOx in the process. The oxidation state of the PdOx was also determined as a function of time during transient tests with and without the reductants. CO and CO/H2 reduced the PdOx more rapidly than C2H4 or H2, which correlated with the superior NOx storage performance with CO and CO/H2. It is proposed that the PdOx reduction was responsible for the gradual drop in NOx storage efficiency on consecutive tests. Consistent with that, the maximum performance could be recovered by oxidizing the catalyst between 700 and 750 °C. Future work includes a similar assessment of zeolite-based LTNAs which are more sulfur tolerant than ceria-based LTNAs.










Similar content being viewed by others
Abbreviations
- C2H4 :
-
ethylene
- Ce:
-
cerium
- CH4 :
-
methane
- CO:
-
carbon monoxide
- CO2 :
-
carbon dioxide
- DOC:
-
diesel oxidation catalyst
- ExitNOx:
-
NOx concentration exiting the reactor
- FGNOx:
-
feedgas NOx entering the reactor
- FTP:
-
federal test procedure
- H2 :
-
hydrogen
- H2O:
-
water
- HC:
-
hydrocarbon
- LNT:
-
lean NOx trap
- LTNA:
-
low temperature NOx adsorber
- N2 :
-
nitrogen
- N2O:
-
nitrous oxide
- NO:
-
nitric oxide
- NOx:
-
oxides of nitrogen = NO +NO2
- NO2 :
-
nitrogen dioxide
- –NO2 :
-
nitrite
- –NO3 :
-
nitrate
- O2 :
-
oxygen
- Pt:
-
platinum
- Pd:
-
palladium
- PGM:
-
platinum group metal
- PM:
-
particulate matter
- SCR:
-
selective catalytic reduction
- TWC:
-
three-way catalyst
References
Lambert, C., Cavataio, G.: Development of the 2010 Ford Diesel Truck Catalyst System. In: Nova, I., Tronconi, E. (eds.) Urea-SCR Technology for deNOx After Treatment of Diesel Exhaust. Springer, New York (2014)
Miyoshi, N., Matsumoto, S., Katoh, K., Tanaka, T., Harada, J., Takahashi, N., Yokota, K., Sgiura, M., Kasahara, K.: Development of New Concept Three-Way Catalyst for Automotive Lean-Burn Engines. SAE paper no. 950809
Coulson, J.E., Brisley, R.J., Keane, O., Phillips, P.R., Mountstevens, E.H.: Thermally Regenerable Nitric Oxide Adsorbent. Patent Application, WO 2008/047170. (2008)
Melville, J.E., Brisley, R.J., Keane, O., Phillips, P.R., Mountstevens, E.H.: Thermally regenerable nitric oxide adsorbent. US Patent US8105559
Chen, H.Y., Shadab, M., Weigert, E., Camm, K., Ballinger, T., Cox, J., Blakeman, P.: Cold Start Concept CSC™: A Novel Catalyst for Cold Start Emission Control. SAE Paper no. 2013-01-0535
Chen, H.Y., Collier, J., Liu, D., Mantarosie, L., Duran-Martin, D., Novak, V., Rajaram, R., Thompsett, D.: Low temperature NOx storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 146(9), 1706–1711 (2016)
Vu, A., Luo, J., Li, J., Epling, W.: Effects of CO on Pd/BEA passive NOx Adsorbers. Catal. Lett. 147(3), 745–750 (2017)
Zheng, Y., Kovarik, L., Engelhard, M., Wang, Y., Wang, Y., Gao, F., Szanyi, J.: Low-temperature Pd/zeolite passive NOx Adsorbers: structure, performance, and adsorption chemistry. J. Phys. Chem. C. 121(29), 15793–15803 (2017)
Mantarosie, L.: An Infrared and XAS Study on NO Adsorption on Pd/Zeolite Under Complex Gas Feed. oral presentation at 2017 NAM conference, Denver, CO
Millo, F., Vezza, D.: Characterization of a New Advanced Diesel Oxidation Catalyst with Low Temperature Storage Capability for LD Diesel. SAE paper no. 2012-02-0373
Ji, Y., Bai, S., Crocker, M.: Al2O3-based passive NOx adsorbers for low temperature applications. App. Catal. B Environ. 170-171, 283–292 (2015)
Ji, Y., Xu, D., Crocker, M., Theis, J., Lambert, C., Bueno-Lopez, A., Harris, D., Scapens, D.: Mn-based mixed-oxides for low temperature NOx adsorber applications. Appl. Cat. A Gen. 567, 90–101 (2018). https://doi.org/10.1016/j.apcata.2018.09.006
Theis, J., Lambert, C.: An assessment of low temperature NOx Adsorbers for cold-start NOx control on diesel engines. Catal. Today. 258(Part 2), 367–377 (2015)
Theis, J.: An assessment of Pt and Pd model catalysts for low temperature NOx adsorption. Catal. Today. 267, 93–109 (2016). https://doi.org/10.1016/j.cattod.2016.01.032
Theis, J., Lambert, C.: The Effects of CO, C2H4, and H2O on the NOx storage Performance of Low Temperature NOx Adsorbers for Diesel Applications. SAE paper no. 2017-01-0942
Theis, J., Lambert, C.: Mechanistic assessment of low temperature NOx adsorbers for cold start NOx control on diesel engines. Catal. Today. 320, 181–195 (2019). https://doi.org/10.1016/j.cattod.2017.12.014
Theis, J., McCabe, R.: The effects of high temperature lean exposure on the subsequent HC conversion of automotive catalysts. Catal. Today. 184(1), 262–270 (2012)
Farrauto, R., Hobson, M., Kennelly, T., Waterman, E.: Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 81(2), 227–237 (1992)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Theis, J.R., Lambert, C.K. Effect of Reductants on the NOx Storage Performance of a Pd/CZO Low Temperature NOx Adsorber. Emiss. Control Sci. Technol. 5, 215–224 (2019). https://doi.org/10.1007/s40825-019-00121-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-019-00121-6