Abstract
This study investigated the prospect of using aqueous mixture of 1-butylpyridinium tetrafluoroborate ([Bpy][BF4]) ionic liquid (IL) and monoethanolamine (MEA) as solvent in post-combustion CO2 capture (PCC) process. This is done by analysis of the process through modelling and simulation. In literature, reported PCC models with a mixture of IL and MEA solvent were developed using equilibrium-based mass transfer approach. In contrast, the model in this study is developed using rate-based mass transfer approach in Aspen Plus®. From the results, the mixed aqueous solvent with 5–30 wt% IL and 30 wt% MEA showed 7%–9% and 12%–27% less specific regeneration energy and solvent circulation rate respectively compared to commonly used 30 wt% MEA solvent. It is concluded that the IL concentration (wt%) in the solvent blend have significant impact on specific regeneration energy and solvent circulation rate. This study is a starting point for further research on technical and economic analysis of PCC process with aqueous blend of IL and MEA as solvent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Background and motivation
Carbon capture and storage (CCS) is considered as an economic and sustainable CO2 abatement technology option for achieving global CO2 emission reduction targets by 2050. The technology involves capturing CO2 from large stationary sources (e.g. fossil fuel-fired power plants and other carbon intensive industries) and transporting them to underground storage sites, namely saline aquifer and depleted oil and gas reserves, where they are either stored permanently and prevented from entering the atmosphere or used for enhanced oil recovery (EOR) purposes (IPCC 2005). Regardless of success with some commercial projects, the technology is still faced with huge development and operating cost especially the carbon capture plant component of the CCS chain which alone is responsible for 75%–80% of the total CCS cost (Davison 2007). This is partly due to 30 wt% monoethanolamine (MEA) used generally in the capture process as solvent. The solvent has unacceptable attributes including relatively high specific regeneration energy up to 4.2 GJ/ton CO2 (Kothandaraman et al. 2009), high solvent circulation rate up to 6 times the flue gas flowrate for coal-fired power plant (Lawal et al. 2012) among others.
As a result, there is need to explore other solvent options that have better attributes in terms of specific regeneration energy and solvent circulation rate. Ionic liquids (ILs) meet these criteria except that they are expensive and have generally slower kinetics compared to aqueous MEA solvent (Huang et al. 2014). However, new solvent formulation obtained by mixing IL and MEA could leverage on the positive attributes of both solvents and result in a more cost-effective and better-performing solvent (Zhang and Rubin 2014).
1.2 Literature review
ILs are classified into conventional room temperature ionic liquid and task specific ionic liquid (TSILs). TSILs are generally more suitable for CO2 absorption at flue gas conditions. More information on different IL categories is available in Ramdin et al. (2012). Shiflett et al. (2010) performed model-based comparison of 1-butyl-3-methylimidazolium acetate [BMIM][Ac] TSIL and commonly used 30 wt% MEA solvent using an equilibrium-based PCC model. Their results showed that the IL solvent have 16% less reboiler duty compared to 30 wt% MEA solvent. They further showed that the capital cost and equipment footprint for the process with IL solvent are 11% and 12% lower than with 30 wt% MEA solvent respectively. However, ILs are highly viscous, expensive and have slow reaction kinetics. These factors seriously discredit their application in the treatment of flue gases.
The difficulties are avoided by mixing ILs with other solvents such as water or alkanolamines as shown through experiments (Camper et al. 2008; Wappel et al. 2010; Yang et al. 2014; Zhang and Rubin 2014). Wappel et al. (2010) reported improved characteristics with a mixture of IL and water although with still slower reaction kinetics and lower absorption capacity than 30 wt% MEA solution. Zhang and Rubin (2014) further showed that mixed IL and methyl diethanolamine (MDEA) solvent was much better as did Camper et al. (2008). Yang et al. (2014) also showed that mixed solvent including 40 wt% 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4] IL and 30 wt% MEA have 37.2% less regeneration energy than the reference 30 wt% MEA solvent.
Huang et al. (2014) performed very detailed comparison of different ILs mixed with MEA and the reference 30 wt% MEA solvent using equilibrium-based mass transfer model of a PCC process. With an aqueous blend of 30 wt% 1-butylpyridinium tetrafluoroborate [Bpy][BF4] IL and 30 wt% MEA solvent, the heat duty and the capture cost is reduced by 15% and 11% respectively compared to the reference 30 wt% MEA solvent.
In conclusion, firstly, aqueous ILs mixed with alkanolamines have better all-round attribute than either IL only or 30 wt% MEA solvent. Secondly, existing PCC models with IL-based solvents are developed using equilibrium-based mass transfer approach. Rate-based mass transfer approach gives more accurate prediction of the process conditions (Peng et al. 2003; Lawal et al. 2009). Finally, none of the studies have investigated the implications of varying the IL concentration in the mixed IL and alkanolamine solvent. Most of the papers used at least 30 wt% IL concentration in the mixed solvent formulation and this means the solvents cost will be significantly high judging from predicted industrial-scale prices of IL solvents.
1.3 Aim and novelty
Literature review summarised in Sect. 1.2 strongly suggests that aqueous blend of IL/MEA solvent is a more efficient solvent for PCC processes compared to either ILs only or 30 wt% MEA solvent. In literature, high IL concentrations (30–40 wt%) in the blended solvent are commonly adopted. However, no detailed technical analysis that justifies the selection of this concentration range has been reported. Also, existing models of PCC process using blended aqueous IL and MEA solvent (Shiflett et al. 2010; Huang et al. 2014) have been derived using equilibrium-based mass transfer approach. Generally, equilibrium-based PCC models are less accurate compared to their rate-based counterparts (Lawal et al. 2009).
This study is aimed at filling this knowledge gap by performing a technical analysis of a PCC process using aqueous blend of IL and MEA solvent through process simulations. The process simulations is carried out using a rate-based model of the process developed with Aspen Plus® and based on a benchmark model obtained from existing publication (Huang et al. 2014).
2 Process description
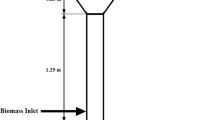
In the process (Fig. 1), flue gas coming from a power plant or other industrial processes is cooled down to about 40 °C before entering the absorber. In the absorber, CO2 in the flue gas is removed through reactions with the solvent. The scrubbed gas is then water washed to recover some of the solvents in the gas phase at the top of the column before they are released into the atmosphere.
Schematic diagram of PCC process (Lawal et al. 2010)
The rich solvent leaving the absorber is heated to about 80 °C in a cross heat exchanger by hot lean solvent, before it enters the stripper. In the stripper, the rich solvent is regenerated by heating it further to about 120 °C at a pressure of about 1.8 atm. The stripper overhead stream (up to 99 wt% CO2) is compressed and transported through pipeline to sequestration sites while the lean solvent from the stripper bottom is pumped back to the absorber.
3 Model development
3.1 Model benchmark
The model by Huang et al. (2014) was used as benchmark for this study. The model was selected because it is the only reported model involving IL-MEA solution as solvent for a PCC process. The model was simulated in Aspen Plus® using RADFRAC equilibrium stage model. Huang et al. (2014) also provided detailed thermodynamic and physical properties of the selected IL and process conditions making it possible for the model to be duplicated.
The flue gas specification (Table 1) is based on the outlet of coke oven combustion chambers at Shanxi Coke Plant in China (Huang et al. 2014). They are assumed to have been desulphurized. The ionic liquid used is 1-butylpyridinium tetrafluoroborate ([Bpy][BF4]). This is because it has more potential for large-scale utility, thanks to its lower cost, toxicity and environmental impact. Other process conditions are given in Tables 1 and 2 (Huang et al. 2014).
3.2 Thermo-physical properties
The phase equilibrium, chemical equilibrium and reaction enthalpy of the MEA–H2O–CO2–IL system was modelled using electrolyte non-random-two-liquid (eNRTL) thermodynamic model available in Aspen Plus®. The thermodynamic model is commonly adopted in modelling MEA scrubbing processes in literature (Lawal et al. 2009, 2010). The default eNRTL parameters and physical property correlations in Aspen plus® for MEA which have been shown to be accurate in published studies such as Lawal et al (2009), (2010) were used for estimating the thermo-physical properties of MEA. On the other hand, new parameters obtained from Huang et al. (2014) were used to estimate the thermo-physical properties of the IL (i.e. [Bpy][BF4]).
3.3 Reaction chemistry
The reaction model is comprised of both equilibrium and rate-controlled reactions (Canepa et al. 2012).
The equilibrium reactions are defined as:
On the other hand, the rate-controlled reactions are defined as:
The equilibrium constant K eq for R1–R3 is estimated as follows:
The reaction rate for the rate-controlled Reactions R4–R7 is determined using the power law expression as follows:
The values of the parameters in Eq. (1) (i.e. \(A\), \(B\), \(C\) and \(D\)) and Eq. (2) (i.e. \(k\) and \(E\)) are given in Table 3.
3.4 Model comparison
There are currently no data for PCC processes using blended aqueous IL and MEA solvent in literature. In this study, Huang et al. (2014) equilibrium-based model was used as benchmark. The model was duplicated and then compared to the original model in Huang et al. (2014). The topology of the duplicate model in Aspen Plus® is shown in Fig. 2. Comparison of results of the duplicate model and the original model (Huang et al. 2014) is shown in Tables 4 and 5. The results show good agreement indicating accurate representation of the Huang et al. (2014) model.
4 Improvement of the model
4.1 Rate-based versus equilibrium-based model
Huang et al. (2014) model duplicated above is an equilibrium-based model developed using RadFrac equilibrium model in Aspen Plus®. In this model, theoretical stages are assumed in which the liquid and vapor phases attain equilibrium characterized by infinitely fast mass transport. Efficiency correlation factors are used to adjust the performance of each stage. In practice, equilibrium is rarely attainable. On the other hand, in rate-based model, actual rate of mass and heat transfer are taken into account. The mass transfer is typically modelled using two film theory.
Peng et al. (2003) and Lawal et al. (2009) among others have compared equilibrium-based and rate-based models of reactive columns. Their results showed that rate-based models of reactive columns give more accurate prediction of the process conditions than their equilibrium-based counterparts. It is therefore concluded that rated-based approach is more suitable for modelling reactive columns. As a result, the Huang et al. (2014) model duplicated in this study is upgraded using rate-based approach so that the model can potentially become more robust and accurate.
4.2 Description of the rate-based model
The packing parameters for the absorber and stripper is given in Table 6. Heat and mass transfer correlations given in Table 7. The columns were sized using generalized pressure drop correlation (Lawal et al. 2012) alongside data from Huang et al. (2014). For the absorber, estimated column diameter was 13.78 m. To confirm that order of magnitude, Aspen estimation was run using the packing sizing method. A diameter of 13.92 m was found which validates the manual estimation. The two methods, manual and Aspen calculation, gives a rough estimate of the column diameter due to some inevitable approximations made during the calculations and are subject to some significant level of uncertainty. As a result, different column diameters around the estimated value were tried. It was found that about 10.5 m diameter was a good compromise between the target 90% capture level and minimum column diameter requirement. A column height of 20 m was chosen for the absorber using the method described in Lawal et al. (2012). The same methods have been used to determine the Stripper’s diameter. After several trial, it is found that a diameter of 9.5 m allows good rate of CO2 in the stripper overhead stream and a good loading of the regenerated solvent.
5 Process analysis
By comparing the rated-based model of the PCC process using aqueous mixture of IL and MEA solvent and the reference 30 wt% MEA solvent, it appears that, as Huang et al. (2014) has showed with equilibrium-based models that IL reduces solvent circulation rate and the energy needed for solvent regeneration. ILs are generally expensive; the prices (lab scale) are over US$1000/kg although BASF predicts that the industrial scale price could drop to <US$40/kg (Ramdin et al. 2012). Ramdin et al. (2012) predicted that the cost of IL would still be a factor of 10–20 higher than MEA even at a price level of <US$40/kg. As a result, Huang et al. (2014) blended IL and MEA solvent formulation involving 30 wt% IL will lead to huge increase in total solvent cost compared to the reference 30 wt% MEA solvent. Consequently, a case study is necessary to explore possibilities of using lower IL concentration in the solvent formulation. In this study, two case studies were developed by varying the concentration of IL in the solvent starting from 0 to 30 wt% in a step of 5 and the impact on reboiler duty and solvent circulation rate studied. The case study was performed using the rate-based model of the PCC process.
5.1 Setup of the case studies
The setup is applicable to the case studies described in Sects. 5.2 and 5.3. In the case studies, the process was simulated using different aqueous solutions of the solvent as follows:
-
1
30 wt% MEA and 0 wt% IL (i.e. base case).
-
2
30 wt% MEA and 5 wt% IL.
-
3
30 wt% MEA and 10 wt% IL.
-
4
30 wt% MEA and 15 wt% IL.
-
5
30 wt% MEA and 20 wt% IL.
-
6
30 wt% MEA and 25 wt% IL.
-
7
30 wt% MEA and 30 wt% IL.
The input conditions given in Tables 2 and 3, packing characteristics given in Table 6 and the column dimensions estimated in Sect. 4.2 were used in all the cases. The capture level was also fixed at 90% for all the cases.
5.2 Impact of IL (wt%) on solvent circulation rate
5.2.1 Justification of the case study
Solvent circulation rate in PCC processes have significant impact on equipment sizes, specific regeneration energy and overall process economics. In this study, the solvent circulation rate is expressed in terms of liquid–gas ratio (L/G ratio); gas flowrate remains the same for all the scenario and as such changes in L/G ratio is directly proportional to the solvent circulation rate. In this case study, the impact of IL concentration in the mixed solvent on the L/G ratio is evaluated. The analysis provides insight on the impacts of operating with different IL concentration on L/G ratio. In addition, it provides a useful guide for selecting appropriate IL concentration for the mixed solvent.
5.2.2 Results and discussions
The result shows reduction in L/G ratio (mol/mol) as IL concentration in the solvent increases (Fig. 3). With 5 wt% IL concentration in the mixed solvent, the L/G ratio reduced by about 11.6%; further increase up to 30 wt% IL concentration achieved about 26.8% reduction in the L/G ratio. The reduction is because the loading capacity of the solvent increases with the addition of IL and as such less solvent circulation is required to achieve the target 90% capture level. Comparing the reductions in L/G ratio achievable at different IL concentrations, it is reckoned that 5 wt% IL concentration is a good compromise considering expected higher cost of IL and reductions in L/G ratio achievable at higher IL concentration. On this basis, it is predicted that 30 wt% IL concentration in the mixed solvent proposed by Huang et al. (2014) may not be economically realistic.
5.3 Impact of IL (wt%) on specific regeneration energy
5.3.1 Justification of the case study
Specific regeneration energy is the energy (reboiler duty) for regenerating loaded solvent per tonne of CO2 stripped from the solvent. It is a common metric for assessing the performance of different PCC processes and the main contributor to overall electricity output penalty for PCC plants added to a fossil fuel-fired power plant (Lucquiaud and Gibbins 2011). It is affected by packing type, CO2 concentration in flue gas, capture level and solvent type (Kothandaraman et al. 2009). Insights from analysis of the impact of different solvent mixtures with varying concentrations of IL on the specific regeneration energy will provide a useful benchmark for comparing the performance of mixed IL ([Bpy][BF4]) and MEA solvent with other solvents. In addition, the result will be an important input for determining the appropriate IL ([Bpy][BF4]) concentration in the mixed IL and MEA solvent.
5.3.2 Results and discussions
The result (Fig. 4) shows that the specific regeneration energy is generally lower for the mixed IL and MEA solvent compared to the base case (i.e. 30 wt% MEA and 0 wt% IL). The specific regeneration energy reduction is attributed to the following factors (Huang et al. 2014):
-
1
Lower heat capacity of IL-MEA hybrid solvent compared to the reference 30 wt% MEA solution.
-
2
Lower solvent flow rate of the IL-MEA hybrid solvent cases compared to the 30 wt% MEA solution case (Sect. 5.2).
-
3
Lower heat of vaporization due to lower amount of water.
Also, it is observed that the specific regeneration energy drops significantly with about 5 wt% IL compared to the base case (i.e. 30 wt% MEA and 0 wt% IL). Further increments in IL wt%, up to 25 wt%, showed very minimal changes in the specific regeneration energy; more noticeable reduction is observed beyond this point. Again, 5 wt% IL appears a good compromise; reductions in specific regeneration energy at higher IL wt% may not be commensurate with the accompanying increase in process economics.
6 Conclusions and recommendations for future research
This study assessed the performance of using a mixed aqueous IL ([Bpy][BF4]) and MEA solvent for CO2 capture in PCC process with the reference 30 wt% MEA solvent as base case. Six (6) compositions of the mixed solvent with varying concentrations of IL and MEA concentration fixed at 30 wt% for all cases were evaluated through process simulations. The highest IL concentration, 30 wt%, showed highest reductions in specific regeneration energy and solvent circulation rate. However, IL is a lot more expensive than MEA. As a result, with 30 wt% IL concentration, the total solvent cost maybe be substantially higher than the base case.
From comparing other compositions, it is found that using 5 wt% IL which reduces the specific regeneration energy and solvent circulation rate by about 7% and 11.5% respectively appears economically competitive with the base case. Therefore, it is recommended that for the IL used in this study (i.e. [Bpy][BF4]), the concentration should be about 5 wt% in the solvent formulation of a mixture of the IL with MEA. Total solvent cost when using higher concentration of the IL may negate the advantages of the IL.
It is recommended that further technical and economic analysis be performed on this process to investigate other conditions that could contribute to the process economics, namely solvent make-up rate, pumping requirements, steam consumption, cooling duty requirements among others. Finally, detailed model validation should be carried out to ensure the model represents the process accurately.
Abbreviations
- C i :
-
Molar concentration of the components (M)
- E :
-
Activation energy (J/kmol)
- K eq :
-
Equilibrium constant
- K :
-
Pre-exponential factor
- R :
-
Reaction rate
- R :
-
Molar gas constant (J/mol K)
- T :
-
Temperature (K)
References
Camper D, Bara J, Gin DL, Noble R (2008) Room-temperature ionic liquid-amine solutions: tunable solvents for efficient and reversible capture of CO2. Ind Eng Chem Res 47:8496–8498
Canepa R, Wang M, Biliyok C, Satta A (2012) Thermodynamic analysis of combined cycle gas turbine power plant with post-combustion CO2 capture and exhaust gas recirculation. J Process Mech Eng 227(2):89–105
Davison J (2007) Performance and costs of power plants with capture and storage of CO2. Energy 32:1163–1176
Huang Y, Zhang X, Zhang X, Dong H, Zhang S (2014) Thermodynamic modelling and assessment of ionic liquid-based CO2 capture processes. Ind Eng Chem Res 53:11805–11817
Bravo JL, Rocha JA, Fair JR (1985) Mass transfer in Gauze Packings. Hydrocarb Process 64:91–95
Bravo JL, Rocha JA, Fair JR (1992) A Comprehensive model in the performance of columns containing structured packings, distillation and absorption, Institution of Chemical Engineers Symposium Series 128. Inst Chem Eng 1:PA48–A507
Inter-gouvernemental Panel on Climate Change (IPCC) (2005) IPCC special report on carbon dioxide capture and storage. Cambridge University Press, Cambridge
Kothandaraman A, Nord L, Bolland O, Herzog HJ, McRae GJ (2009) Comparison of solvents for post-combustion capture of CO2 by chemical absorption. Energy Procedia 1:1373–1380
Lawal A, Wang M, Stephenson P, Yeung H (2009) Dynamic modelling of CO2 absorption for post-combustion capture in coal-fired power plants. Fuel 88(12):2455–2462
Lawal A, Wang M, Stephenson P, Koumpouras G, Yeung H (2010) Dynamic modelling and analysis of post-combustion CO2 chemical absorption process for coal-fired power plants. Fuel 89(10):2791–2801
Lawal A, Wang M, Stephenson P, Obi O (2012) Demonstrating full-scale post-combustion CO2 capture for coal-fired power plants through dynamic modelling and simulation. Fuel 101:115–128
Lucquiaud M, Gibbins J (2011) On the integration of CO2 capture with coal-fired power plants: a methodology to assess and optimise solvent-based post-combustion capture systems. Chem Eng Res Des 89:1553–1571
Onda K, Takeuchi H, Okumoto Y (1968) Mass transfer coefficients between gas and liquid phases in packed columns. J Chem Eng Jpn 1:56–62
Peng J, Edgar TF, Eldridge RB (2003) Dynamic rate-based and equilibrium models for a packed reactive distillation column. Chem Eng Sci 58:2671–2680
Ramdin M, de Loos TW, Vlugt TJH (2012) State-of-the-art of CO2 capture with ionic liquids. Ind Eng Chem Res 51:8149–8177
Shiflett MB, Drew DW, Cantini RA, Yokozeki A (2010) Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy Fuels 24:5781–5789
Stichlmair J, Bravo JL, Fair JR (1989) General model for prediction of pressure drop and capacity of countercurrent gas/liquid packed columns. Gas Sep Purif 3:19
Wappel D, Gronald G, Kalb R, Draxler J (2010) Ionic liquids for post-combustion CO2 absorption. Int J Greenh Gas Control 4(3):486–494
Yang J, Yu X, Yan J, Tu ST (2014) CO2 capture using amine solution mixed with ionic liquid. Ind Eng Chem Res 53:2790–2799
Zhang H, Rubin ES (2014) Systems analysis of ionic liquids for post-combustion CO2 capture at coal-fired power plants. Energy Procedia 63:1321–1328
Acknowledgements
The authors from University of Hull acknowledge financial support from EU FP7 (Reference: PIRSES-GA-2013-612230).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zacchello, B., Oko, E., Wang, M. et al. Process simulation and analysis of carbon capture with an aqueous mixture of ionic liquid and monoethanolamine solvent. Int J Coal Sci Technol 4, 25–32 (2017). https://doi.org/10.1007/s40789-016-0150-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-016-0150-1