Abstract
Purpose of Review
Prenatal intervention for congenital diseases could potentially halt or reverse devastating co-morbidities. Particle-based therapy has emerged as safe option for in-utero treatment delivery. Here, we focus on outlining the current state of knowledge regarding the application of particle-based fetal therapy in the management two structural diseases: congenital diaphragmatic hernia (CDH) and myelomeningocele (MMC).
Recent Findings
Intravenous or intra-amniotic injection of particles distribute to various tissues, including the lung, liver, and can cover surfaces such as the spine. They can be modified to target specific tissues and can carry various loads, including protein and genetic material.
Summary
In CDH, microRNA loaded particles have successfully reversed the CDH phenotype in animal models. In MMC, particle delivery has resulted in tissue growth and coverage of the spinal defect. Further research is needed for clinical translation in larger animal models and to assess long-term functional outcomes following particle-based interventions.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Zu H, Gao D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. AAPS J. 2021;23(4):78.
Butt MH, et al. Appraisal for the potential of viral and nonviral vectors in gene therapy: a review. Genes (Basel). 2022;13(8):1370.
Lee JH, Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci. 2015;125:75–84.
Yao Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193.
Swingle KL, et al. Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J Control Release. 2022;341:616–33.
Riley RS, et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci Adv. 2021;7(3):eaba1028.
Bose SK, et al. In utero adenine base editing corrects multi-organ pathology in a lethal lysosomal storage disease. Nat Commun. 2021;12(1):4291.
Ricciardi AS, et al. In utero nanoparticle delivery for site-specific genome editing. Nat Commun. 2018;9(1):2481.
• Deprest JA, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. 2021;385(2):107–18. Findings from this important randomized clinical trial provided evidence that the use of FETO for CDH resulted in significant benefit over observation or expectant management alone.
Montalva L, et al. Neurodevelopmental impairment in children with congenital diaphragmatic hernia: not an uncommon complication for survivors. J Pediatr Surg. 2020;55(4):625–34.
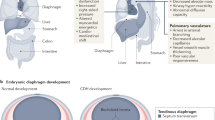
Montalva L, Zani A. Assessment of the nitrofen model of congenital diaphragmatic hernia and of the dysregulated factors involved in pulmonary hypoplasia. Pediatr Surg Int. 2019;35(1):41–61.
Greer JJ. Current concepts on the pathogenesis and etiology of congenital diaphragmatic hernia. Respir Physiol Neurobiol. 2013;189(2):232–40.
Goncalves AN, Correia-Pinto J, Nogueira-Silva C. ROBO2 signaling in lung development regulates SOX2/SOX9 balance, branching morphogenesis and is dysregulated in nitrofen-induced congenital diaphragmatic hernia. Respir Res. 2020;21(1):302.
Mous DS, et al. Pulmonary vascular development in congenital diaphragmatic hernia. Eur Respir Rev. 2018;27(147).
Wynn J, Yu L, Chung WK. Genetic causes of congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19(6):324–30.
Cushing L, et al. The roles of microRNAs and protein components of the microRNA pathway in lung development and diseases. Am J Respir Cell Mol Biol. 2015;52(4):397–408.
Pereira-Terra P, et al. Unique tracheal fluid microRNA signature predicts response to FETO in patients with congenital diaphragmatic hernia. Ann Surg. 2015;262(6):1130–40.
Chan YC. MicroRNA regulation of angiogenesis. In: Sen CK, editor. MicroRNA in regenerative medicine. Academic Press; 2023. p. 539–72.
• Ullrich SJ, et al. In utero delivery of miRNA induces epigenetic alterations and corrects pulmonary pathology in congenital diaphragmatic hernia. Mol Ther Nucleic Acids. 2023;32:594–602. This study shows promise that the delivery of particle therapy carrying miRNA200b reverses the CDH phenotype and is a potentially translatable treatment.
Mandl HK, et al. Optimizing biodegradable nanoparticle size for tissue-specific delivery. J Control Release. 2019;314:92–101.
Kauffman AC, et al. Tunability of biodegradable poly(amine- co-ester) polymers for customized nucleic acid delivery and other biomedical applications. Biomacromolecules. 2018;19(9):3861–73.
Ambegia E, et al. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim Biophys Acta. 2005;1669(2):155–63.
Gao Y, et al. Multifunctional nanoparticle for cancer therapy. MedComm (2020). 2023;4(1):e187.
• Luks VL, et al. Surface conjugation of antibodies improves nanoparticle uptake in bronchial epithelial cells. PLoS One. 2022;17(4):e0266218. This study highlights how particles can be edited to make them tissue and cell specific.
Ullrich SJ, et al. Nanoparticles for delivery of agents to fetal lungs. Acta Biomater. 2021;123:346–53.
Khoshgoo N, et al. MicroRNA-200b regulates distal airway development by maintaining epithelial integrity. Sci Rep. 2017;7(1):6382.
Manning SM, Jennings R, Madsen JR. Pathophysiology, prevention, and potential treatment of neural tube defects. Ment Retard Dev Disabil Res Rev. 2000;6(1):6–14.
Davis BE, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41(4):186–91.
Farrelly JS, et al. Alginate microparticles loaded with basic fibroblast growth factor induce tissue coverage in a rat model of myelomeningocele. J Pediatr Surg. 2019;54(1):80–5.
Adzick NS, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004.
Espinoza J, et al. Two-port, exteriorized uterus, fetoscopic meningomyelocele closure has fewer adverse neonatal outcomes than open hysterotomy closure. Am J Obstet Gynecol. 2021;225(3):327e1–9.
Keil C, et al. Implementation and assessment of a laparotomy-assisted three-port fetoscopic spina bifida repair program. J Clin Med. 2023;12(15):5151.
Sanz Cortes M, et al. Ambulation after in-utero fetoscopic and open spina bifida repair: predictors for ambulation at 30 months. Ultrasound Obstet Gynecol. 2024. https://doi.org/10.1002/uog.27589. Epub ahead of print. PMID: 38243917.
Watanabe M, Kim AG, Flake AW. Tissue engineering strategies for fetal myelomeningocele repair in animal models. Fetal Diagn Ther. 2015;37(3):197–205.
Dionigi B, et al. Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. J Pediatr Surg. 2015;50(6):1037–41.
Shieh HF, et al. Transamniotic stem cell therapy (TRASCET) in a rabbit model of spina bifida. J Pediatr Surg. 2019;54(2):293–6.
Turner CG, et al. Intra-amniotic delivery of amniotic-derived neural stem cells in a syngeneic model of spina bifida. Fetal Diagn Ther. 2013;34(1):38–43.
Chen YJ, et al. Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. J Pediatr Surg. 2017. https://doi.org/10.1016/j.jpedsurg.2017.10.040. Epub ahead of print. PMID: 29096888.
Galganski LA, et al. In utero treatment of myelomeningocele with placental mesenchymal stromal cells - selection of an optimal cell line in preparation for clinical trials. J Pediatr Surg. 2020;55(9):1941–6.
Stokes SC, et al. Impact of gestational age on neuroprotective function of placenta-derived mesenchymal stromal cells. J Surg Res. 2022;273:201–10.
Wang A, et al. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4(6):659–69.
Freedman-Weiss MR, et al. Engineering alginate microparticles for optimized accumulation in fetal rat myelomeningocele. J Pediatr Surg. 2022;57(3):544–50.
Maassel NL, et al. Intra-amniotic injection of poly(lactic-co-glycolic acid) microparticles loaded with growth factor: effect on tissue coverage and cellular apoptosis in the rat model of myelomeningocele. J Am Coll Surg. 2022;234(6):1010–9.
Ching SH, Bansal N, Bhandari B. Alginate gel particles-a review of production techniques and physical properties. Crit Rev Food Sci Nutr. 2017;57(6):1133–52.
Kopac T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: a critical review. Int J Biol Macromol. 2021;169:290–301.
Acknowledgements
Current Stem Cell Reports is grateful to Dr. Graça Almeida-Porada, for her review of this manuscript.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Data review: R.R., D.S.; Resources: R.R., D.S.; Writing - original draft: R.R.; Writing - review & editing: R.R., D.S.
Corresponding author
Ethics declarations
Conflict of Interest
Rachel Rivero declares that she has no conflict of interest. David Stitelman is an editor for Current Stem Cell Reports. David Stitelman is an inventor on this patent. This technology is described: U.S.S.N. 62/481,562, U.S.S.N. 62/489,377, and U.S.S.N. 62/589,275.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Animal Care and Use Committee of Yale University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rivero, R., Stitelman, D.H. Prenatal Therapy for Congenital Diaphragmatic Hernia and Myelomeningocele: Advances in Particle-Based Delivery. Curr Stem Cell Rep (2024). https://doi.org/10.1007/s40778-024-00237-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40778-024-00237-8