Abstract
Purpose of Study
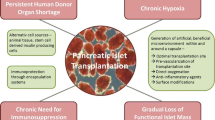
Although the current treatment option of exogenous insulin administration for type 1 diabetes mellitus (T1DM) corrects hyperglycemia, it has its own limitations and complications in long-term use. Thus, alternative approaches such as immune therapies, glucose transporter inhibitors, gastro-enteric protein-hormone pathway modulators, and cell- and tissue-based therapies are being developed. Among these therapies, islet transplantation has been shown to be a more physiological means of treating type 1 diabetes. However, the shortage of donor tissues and the use of immunosuppressive agents have led to the development of immune isolation techniques such as cell encapsulation.
Recent findings
Although macroencapsulation of islets has been shown with some success, microencapsulation mostly with permselectively coated alginate hydrogel has been demonstrated to be superior among the variety of developed encapsulation technologies including nanoencapsulation and thus, has led to several clinical trials. While microencapsulated islet transplantation has shown promise in correcting the pathological symptoms of T1DM, the technology still requires improvement in a few areas in order to achieve sustained performance in long-term application.
Summary
Some approaches suggested for improvement include incorporation of immunomodulatory stem cells such as mesenchymal stem cells, substitution of current crosslinking agents with stable safe divalent cations, improving the chemistry of alginate by adding functional groups, and including extracellular matrix (ECM) components of the pancreas in the encapsulated islet construct. With thorough investigation and improvement on the pitfalls of the technology, and more clinical trials, the microencapsulation technology would provide a viable option for a sustainable and more physiological means of insulin delivery in T1DM.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bullard KM, et al. Prevalence of diagnosed diabetes in adults by diabetes type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):359–61.
International Diabetes Federation, IDF Diabetes Atlas. 2019: Brussels, Belgium.
CDC report, National Diabetes Statistics Report. 2020: Atlanta, GA, USA.
Diagnosis and classification of diabetes mellitus. Diabetes Care, 2009. 32 Suppl 1(Suppl 1): p. S62-7.
Mobasseri M, et al. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10(2):98–115.
Maahs DM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–97.
JDRF, Type 1 Diabetes Facts 2020.
Liese AD, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–8.
Dabelea D, et al. Incidence of diabetes in youth in the United States. Jama. 2007;297(24):2716–24.
Banting FG, Best CH. Pancreatic extracts. 1922. J Lab Clin Med. 1990;115(2):254–72 This is the original paper that describes the discovery of insulin by Banting and Best in Canada.
Pathak V, et al. Therapies for type 1 diabetes: current scenario and future perspectives. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551419844521.
REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ 2017. 356: p. j1285. https://doi.org/10.1136/bmj.j1285.
Abramson A, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363(6427):611–5.
Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for type 1 diabetes effective? Diabet Med. 2019;36(3):279–86.
Kambe N, et al. Impact of newly developed, next-generation artificial endocrine pancreas. J Med Invest. 2015;62(1-2):41–4.
Slover RH, et al. Accuracy of a fourth-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2018;20(9):576–84.
Jacobs PG, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18(11):1110–9.
Haidar A, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(8):595–604.
Weisman A, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–12.
Bergenstal RM, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407–8.
Bekiari E, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. Bmj. 2018;361:k1310.
Anonymous. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. The Canadian-European Randomized Control Trial Group. Diabetes, 1988. 37(11): p. 1574-82.
Russell G, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 Suppl 3):16–22.
Martin S, et al. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care. 1992;15(1):66–74.
Ludvigsson J. Therapies to preserve β-cell function in type 1 diabetes. Drugs. 2016;76(2):169–85.
Gitelman SE, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59(6):1153–61.
Moran A, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15.
Visperas A, Vignali DA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol. 2016;197(10):3762–70.
Takemoto N, et al. Coaggregates of regulatory T cells and islet cells allow long-term graft survival in liver without immunosuppression. Transplantation. 2015;99(5):942–7.
Bluestone JA, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315–189.
Singha S, et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017;12(7):701–10.
Clemente-Casares X, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530(7591):434–40.
Millar PJ, et al. Beneficial metabolic actions of a stable GIP agonist following pre-treatment with a SGLT2 inhibitor in high fat fed diabetic mice. Mol Cell Endocrinol. 2016;420:37–45.
van Baar MJB, et al. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543–56.
Tahara A, et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66(7):975–87.
Cheng ST, et al. The effects of empagliflozin, an SGLT2 inhibitor, on pancreatic β-cell mass and glucose homeostasis in type 1 diabetes. PLoS One. 2016;11(1):e0147391.
McCrimmon RJ, Henry RR. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia. 2018;61(10):2126–33.
Pathak VI, N, Flatt PR, The enteroinsular axis – contribution to obesity-diabetes and its treatments, in nutrition and diabetes: pathophysiology and management, E.C.D.-J. Opara, S., Editor. CRC Press: Boca Raton, FL. 2019, p. 41-56.
Frandsen CS, et al. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. 2015;38(12):2250–7.
Traina AN, et al. Once-weekly exenatide as adjunct treatment of type 1 diabetes mellitus in patients receiving continuous subcutaneous insulin infusion therapy. Can J Diabetes. 2014;38(4):269–72.
Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3(3):241–51.
Pathak NM, et al. Stable oxyntomodulin analogues exert positive effects on hippocampal neurogenesis and gene expression as well as improving glucose homeostasis in high fat fed mice. Mol Cell Endocrinol. 2015;412:95–103.
Irwin N, et al. Sustained treatment with a stable long-acting oxyntomodulin analogue improves metabolic control and islet morphology in an experimental model of type 1 diabetes. Diabetes Obes Metab. 2015;17(9):887–95.
Khan D, et al. Differential expression of glucagon-like peptide-2 (GLP-2) is involved in pancreatic islet cell adaptations to stress and beta-cell survival. Peptides. 2017;95:68–75.
Khan D, et al. Islet distribution of peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol Cell Endocrinol. 2016;436:102–13.
Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther. 2020;11(1):275.
de Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:631463.
Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–64.
Taylor CJ, et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11(2):147–52.
Kondo Y, et al. iPSC technology-based regenerative therapy for diabetes. Journal of Diabetes Investigation. 2018;9(2):234–43.
El Khatib MM, et al. β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther. 2015;22(5):430–8.
Figueiredo C, Blasczyk R. A future with less HLA: potential clinical applications of HLA-universal cells. Tissue Antigens. 2015;85(6):443–9.
Koga K, Wang B, Kaneko S. Current status and future perspectives of HLA-edited induced pluripotent stem cells. Inflammation and Regeneration. 2020;40(1):23.
Lee J, et al. Abrogation of HLA surface expression using CRISPR/Cas9 genome editing: a step toward universal T cell therapy. Sci Rep. 2020;10(1):17753.
Gornalusse GG, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35(8):765–72.
Swanson KJ, et al. Incidence and outcomes of significant weight changes after pancreas transplant alone. Transplant Direct. 2020;6(3):e539.
Robertson P, et al. Pancreas transplantation in type 1 diabetes. Diabetes Care. 2004;27(Suppl 1):S105.
Hampson FA, et al. Pancreatic transplantation: surgical technique, normal radiological appearances and complications. Insights Imaging. 2010;1(5-6):339–47.
Kelly WD, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61(6):827–37.
Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15(1):112–8.
Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Clin Transpl. 2008:45–56.
Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;8(1):6–16.
Dholakia S, et al. Advances in pancreas transplantation. J R Soc Med. 2016;109(4):141–6.
Liong SY, et al. Complications following pancreatic transplantations: imaging features. Abdom Imaging. 2011;36(2):206–14.
Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72(2):175–86.
Sutherland DE, et al. Transplantation of dispersed pancreatic islet tissue in humans: autografts and allografts. Diabetes. 1980;29(Suppl 1):31–44.
Ricordi C, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–20.
Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8 This paper may be described as the dawn of new era of clinical islet transplantation as it reported extended periods of insulin independence for the first time in several patients after intraportal allo-islet transplantation.
Johansson H, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–62.
Koh A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4):465–71.
Bottino R, et al. The Future of Islet Transplantation Is Now. Front Med (Lausanne). 2018;5:202.
McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2(7):a007823.
Yeh HC, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–47.
Jacobson AM, et al. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care. 2013;36(10):3131–8.
Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther. 2018;9(1):349–61.
Panero F, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32(2):301.
Johansson BL, et al. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia. 1996;39(6):687–95.
Johansson BL, et al. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type 1 diabetes mellitus. Diabet Med. 2000;17(3):181–9.
Johansson BL, et al. Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab. 1993;77(4):976–81.
Johansson BL, Linde B, Wahren J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35(12):1151–8.
Johansson BL, Sjöberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35(2):121–8.
Hills CE, Brunskill NJ. C-Peptide and its intracellular signaling. Rev Diabet Stud. 2009;6(3):138–47.
Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50(3):503–9.
Sima AA, et al. Sequential abnormalities in type 1 diabetic encephalopathy and the effects of C-peptide. Rev Diabet Stud. 2009;6(3):211–22.
Luppi P, Cifarelli V, Wahren J. C-peptide and long-term complications of diabetes. Pediatr Diabetes. 2011;12(3 Pt 2):276–92.
Wahren J, Kallas Å, Sima AAF. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes. 2012;61(4):761–72.
Vantyghem MC, et al. Effects of non-steroid immunosuppressive drugs on insulin secretion in transplantation. Ann Endocrinol (Paris). 2007;68(1):21–7.
Froud T, et al. Resolution of neurotoxicity and beta-cell toxicity in an islet transplant recipient following substitution of tacrolimus with MMF. Cell Transplant. 2006;15(7):613–20.
Zhang N, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006;55(9):2429–36.
Niclauss N, et al. Rapamycin impairs proliferation of transplanted islet β cells. Transplantation. 2011;91(7):714–22.
Hyder A, Laue C, Schrezenmeir J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005;19(4):541–6.
Mense MG, Rosol TJ, Chapter 35 - endocrine pancreas, in Boorman's pathology of the rat (second edition), A.W. Suttie, Editor. Academic Press: Boston.2018, p. 695-704.
Opara EC, Kendall WF Jr. Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther. 2002;2(5):503–11.
de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45(2):159–73.
de Vos P, van Schilfgaarde R. In: Kühtreiber WM, Lanza RP, Chick WL, editors. Biocompatibility issues, in cell encapsulation technology and therapeutics. Boston, MA: Birkhäuser Boston; 1999. p. 63–75.
Bisceglie V. Über die antineoplastische Immunität. Zeitschrift für Krebsforschung. 1934;40(1):122–40.
Aebischer P, et al. Functional recovery in hemiparkinsonian primates transplanted with polymer-encapsulated PC12 cells. Exp Neurol. 1994;126(2):151–8.
Wong H, Chang TM. Bioartificial liver: implanted artificial cells microencapsulated living hepatocytes increases survival of liver failure rats. Int J Artif Organs. 1986;9(5):335–6.
Cieslinski DA, David Humes H. Tissue engineering of a bioartificial kidney. Biotechnol Bioeng. 1994;43(7):678–81.
Chang TM. Semipermeable microcapsules. Science. 1964;146(3643):524–5.
Koo J, Chang TM. Secretion of erythropoietin from microencapsulated rat kidney cells: preliminary results. Int J Artif Organs. 1993;16(7):557–60.
Prehn RT, Weaver JM, Algire GH. The diffusion-chamber technique applied to a study of the nature of homograft resistance. J Natl Cancer Inst. 1954;15(3):509–17.
Elliott RB, et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005;37(1):466–9.
Dufrane D, et al. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81(9):1345–53.
Abalovich AG, et al. Pig pancreatic islet transplantation into spontaneously diabetic dogs. Transplant Proc. 2009;41(1):328–30.
Wang T, et al. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation. 2008;85(3):331–7.
Meyer T, Höcht B, Ulrichs K. Xenogeneic islet transplantation of microencapsulated porcine islets for therapy of type I diabetes: long-term normoglycemia in STZ-diabetic rats without immunosuppression. Pediatr Surg Int. 2008;24(12):1375–8.
Foster JL, et al. Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation. 2007;83(11):1440–8.
Soon-Shiong P, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343(8903):950–1.
Tuch BE, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–9.
Calafiore R, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–8.
Scharp DW, Mason NS, Sparks RE. Islet immuno-isolation: the use of hybrid artificial organs to prevent islet tissue rejection. World J Surg. 1984;8(2):221–9.
Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14(9):1107–11.
Colton CK, Avgoustiniatos ES. Bioengineering in development of the hybrid artificial pancreas. J Biomech Eng. 1991;113(2):152–70.
Chick WL, Like AA, Lauris V. Beta cell culture on synthetic capillaries: an artificial endocrine pancreas. Science. 1975;187(4179):847–9.
Maki T, et al. Treatment of diabetes by xenogeneic islets without immunosuppression. Use of a vascularized bioartificial pancreas. Diabetes. 1996;45(3):342–7.
Maki T, et al. Treatment of severe diabetes mellitus for more than one year using a vascularized hybrid artificial pancreas. Transplantation. 1993;55(4):713–7 discussion 717-8.
Lacy PE, et al. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254(5039):1782–4.
Jain K, et al. Glucose control and long-term survival in biobreeding/Worcester rats after intraperitoneal implantation of hydrophilic macrobeads containing porcine islets without immunosuppression. Transplantation. 1999;68(11):1693–700.
Scharp DW, et al. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes. 1994;43(9):1167–70 This is one of the first reports of encapsulation as an effective strategy for islet immunoisolation in clinical transplantation.
Storrs R, et al. Preclinical development of the islet sheet. Ann N Y Acad Sci. 2001;944:252–66.
van Suylichem PT, et al. Insulin secretion by rat islet isografts of a defined endocrine volume after transplantation to three different sites. Diabetologia. 1992;35(10):917–23.
de Vos P, et al. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27(32):5603–17.
Iwata H, et al. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes. 1989;38(Suppl 1):224–5.
Cruise GM, et al. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8(3):293–306.
Zielinski BA, Aebischer P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 1994;15(13):1049–56.
Haug A, Larsen B, A study on the constitution of alginic acid by partial acid hydrolysis, in Proceedings of the fifth international seaweed symposium, Halifax, August 25–28, 1965, E.G. Young and J.L. McLachlan, Editors. Pergamon. 1966, p. 271-277.
Enck, K., et al., Effect of alginate matrix engineered to mimic the pancreatic microenvironment on encapsulated islet function. Biotechnol Bioeng, 2020.
Zhang J, et al. Influence of divalent cations on the biofouling behaviors of alginate hydrogels. Biomed Mater. 2019;15(1):015003.
Mørch YA, et al. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7(5):1471–80.
Tao H, et al. Inconceivable hypokalemia: a case report of acute severe barium chloride poisoning. Case Reports in Medicine. 2016;2016:2743134.
Bhoelan BS, et al. Barium toxicity and the role of the potassium inward rectifier current. Clinical Toxicology. 2014;52(6):584–93.
Struyk AF, Cannon SC. Paradoxical depolarization of BA2+- treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle & Nerve. 2008;37(3):326–37.
Brady SA, et al. Optimisation of a novel glass-alginate hydrogel for the treatment of intracranial aneurysms. Carbohydr Polym. 2017;176:227–35.
Kang S-M, et al., Alginate microencapsulation for three-dimensional In vitro cell culture. ACS Biomaterials Science & Engineering, 2020.
Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–10 This is the first report that described microencapsulated islets as a bioartificial pancreas and demonstrated its efficacy in streptozotocin-induced diabetic rats.
Rabanel JM, et al. Progress technology in microencapsulation methods for cell therapy. Biotechnol Prog. 2009;25(4):946–63.
Wolters GH, et al. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 1991;3(4):281–6.
Opara EC, McQuilling JP, Farney AC. Microencapsulation of pancreatic islets for use in a bioartificial pancreas. Methods Mol Biol. 2013;1001:261–6.
Omer A, et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–8.
Liaudanskaya V, et al. Assessing the impact of electrohydrodynamic jetting on encapsulated cell viability, proliferation, and ability to self-assemble in three-dimensional structures. Tissue Eng Part C Methods. 2015;21(6):631–8.
Ma M, et al. Core-shell hydrogel microcapsules for improved islets encapsulation. Adv Healthc Mater. 2013;2(5):667–72.
Weibel DB, Diluzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Microbiol. 2007;5(3):209–18.
Choi JK, et al. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials. 2014;35(19):5122–8.
Headen DM, et al. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater. 2014;26(19):3003–8.
Weaver JD, et al. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am J Transplant. 2019;19(5):1315–27.
Enck K, et al. Design of an adhesive film-based microfluidic device for alginate hydrogel-based cell encapsulation. Ann Biomed Eng. 2020;48(3):1103–11.
Tendulkar S, et al. A scalable microfluidic device for the mass production of microencapsulated islets. Transplant Proc. 2011;43(9):3184–7.
Tendulkar S, et al. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012;14(3):461–9.
Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–65.
Ashimova, A., et al., Cell encapsulation within alginate microcapsules: immunological challenges and outlook. Frontiers in Bioengineering and Biotechnology, 2019. 7(380).
Lanza RP, et al. Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres. Transplantation. 1995;59(10):1377–84.
Cui H, et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88(2):160–9.
Paredes-Juarez GA, et al. DAMP production by human islets under low oxygen and nutrients in the presence or absence of an immunoisolating-capsule and necrostatin-1. Sci Rep. 2015;5:14623.
Vaithilingam V, et al. Characterisation of the xenogeneic immune response to microencapsulated fetal pig islet-like cell clusters transplanted into immunocompetent C57BL/6 mice. PLoS One. 2013;8(3):e59120.
Vaithilingam V, et al. Effect of prolonged gelling time on the intrinsic properties of barium alginate microcapsules and its biocompatibility. J Microencapsul. 2011;28(6):499–507.
Kendall WF Jr, Opara EC. Polymeric materials for perm-selective coating of alginate microbeads. Methods Mol Biol. 2017;1479:95–109.
de Vos P, Hoogmoed CG, Busscher HJ. Chemistry and biocompatibility of alginate-PLL capsules for immunoprotection of mammalian cells. J Biomed Mater Res. 2002;60(2):252–9.
Sittadjody S, et al. Encapsulation of mesenchymal stem cells in 3D ovarian cell constructs promotes stable and long-term hormone secretion with improved physiological outcomes in a syngeneic Rat model. Ann Biomed Eng. 2020;48(3):1058–70.
Sittadjody S, et al. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun. 2017;8(1):1858.
Darrabie MD, Kendall WF Jr, Opara EC. Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials. 2005;26(34):6846–52.
Pareta R, et al. Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas. 2014;43(4):605–13.
McQuilling JP, et al. Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. Biomater Sci. 2017;5(12):2437–47.
Krol S, et al. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6(9):1933–9.
Kozlovskaya V, et al. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv Funct Mater. 2012;22(16):3389–98.
Zhi ZL, Khan F, Pickup JC. Multilayer nanoencapsulation: a nanomedicine technology for diabetes research and management. Diabetes Res Clin Pract. 2013;100(2):162–9.
Wilson JT, et al. Noncovalent cell surface engineering with cationic graft copolymers. J Am Chem Soc. 2009;131(51):18228–9.
Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8(7):1940–8.
Luan NM, Teramura Y, Iwata H. Layer-by-layer co-immobilization of soluble complement receptor 1 and heparin on islets. Biomaterials. 2011;32(27):6487–92.
Tatsumi K, et al. The non-invasive cell surface modification of hepatocytes with PEG-lipid derivatives. Biomaterials. 2012;33(3):821–8.
Teramura Y, Kaneda Y, Iwata H. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials. 2007;28(32):4818–25.
Shih H, Mirmira RG, Lin CC. Visible light-initiated interfacial thiol-norbornene photopolymerization for forming islet surface conformal coating. J Mater Chem B. 2015;3(2):170–5.
Lee DY, et al. Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules. J Biomed Mater Res. 2002;62(3):372–7.
Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67-68:35–73.
Teramura Y, Iwata H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev. 2010;62(7-8):827–40.
Zhi ZL, et al. Polysaccharide multilayer nanoencapsulation of insulin-producing beta-cells grown as pseudoislets for potential cellular delivery of insulin. Biomacromolecules. 2010;11(3):610–6.
Teramura Y, Iwata H. Improvement of graft survival by surface modification with poly(ethylene glycol)-lipid and urokinase in intraportal islet transplantation. Transplantation. 2011;91(3):271–8.
Kizilel S, et al. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A. 2010;16(7):2217–28.
Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27(34):5828–35.
Veerabadran NG, et al. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol Biosci. 2007;7(7):877–82.
Vaithilingam V, Bal S, Tuch BE. Encapsulated islet transplantation: where do we stand? Rev Diabet Stud. 2017;14(1):51–78.
O'Sullivan ES, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32(6):827–44.
Phelps EA, et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24(1):64–70 2.
Hwang PT, et al. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016;3(1):28.
Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90(10):1054–62.
Ludwig B, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A. 2013;110(47):19054–8.
Ludwig B, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci U S A. 2012;109(13):5022–7.
Neufeld T, et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One. 2013;8(8):e70150.
Farina M, et al. Cell encapsulation: overcoming barriers in cell transplantation in diabetes and beyond. Adv Drug Deliv Rev. 2019;139:92–115.
Berman DM, et al. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65(5):1350–61.
An D, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci U S A. 2018;115(2):E263–e272.
Dufrane DM, M, Goffin E, Aouassar N, Gianello P, Vandeleene B. A simple and safe clinical procedure for human encapsulated islet transplantation in the subcutaneous tissue for diabetes treatment. Transplantation. 2013;96:S43.
Espona-Noguera A, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11):597.
Kepsutlu B, et al. Design of bioartificial pancreas with functional micro/nano-based encapsulation of islets. Curr Pharm Biotechnol. 2014;15(7):590–608.
Skrzypek K, et al. Pancreatic islet macroencapsulation using microwell porous membranes. Scientific Reports. 2017;7(1):9186.
Dufrane D, Gianello P. Macro- or microencapsulation of pig islets to cure type 1 diabetes. World J Gastroenterol. 2012;18(47):6885–93.
Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8(4):270–80.
Strand BL, et al. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10(3):263–75.
Sun Y, et al. Normalization of diabetes in spontaneously diabetic cynomolgus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98(6):1417–22.
Duvivier-Kali VF, et al. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50(8):1698–705.
Shimoda M, Matsumoto S. Update regarding xenotransplantation in Japan. Xenotransplantation. 2019;26(1):e12491.
Basta G, Montanucci P, Calafiore R, Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Investig, 2020.
Qi M. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv Med. 2014;2014:429710.
Hill RS, et al. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann N Y Acad Sci. 1997;831:332–43.
Soon-Shiong P, et al. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation. 1992;54(5):769–74.
Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21.
Sato Y, et al. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem. 2011;286(14):12524–32.
Hilbrands R, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes. 2009;58(10):2267–76.
De Vos P, et al. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40(3):262–70.
Paredes-Juarez GA, et al. A technology platform to test the efficacy of purification of alginate. Materials (Basel). 2014;7(3):2087–103.
Krishnan R, et al. Immunological challenges facing translation of alginate encapsulated porcine islet xenotransplantation to human clinical trials. Methods Mol Biol. 2017;1479:305–33.
Dorrington MG, Fraser IDC. NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. 2019;10:705.
Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316.
Flo TH, et al. Involvement of toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J Biol Chem. 2002;277(38):35489–95.
Hu S, de Vos P. Polymeric approaches to reduce tissue responses against devices applied for islet-cell encapsulation. Front Bioeng Biotechnol. 2019;7:134.
Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–5.
Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8(1):11–3.
Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–89.
Fullagar B, et al. Nano-encapsulation of bilirubin in pluronic F127-chitosan improves uptake in β cells and increases islet viability and function after hypoxic stress. Cell Transplant. 2017;26(10):1703–15.
Suvarnapathaki S, et al. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Materials. 2019;11(1):65.
Mouré A, et al. Extracellular hemoglobin combined with an O(2) -generating material overcomes O(2) limitation in the bioartificial pancreas. Biotechnol Bioeng. 2019;116(5):1176–89.
Stiegler P, et al. Prevention of oxidative stress in porcine islet isolation. J Artif Organs. 2010;13(1):38–47.
Wang H, et al. Bilirubin can induce tolerance to islet allografts. Endocrinology. 2006;147(2):762–8.
Adin CA, et al. Physiologic doses of bilirubin contribute to tolerance of islet transplants by suppressing the innate immune response. Cell Transplant. 2017;26(1):11–21.
Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005;288(4):F778–84.
Krishnan RK, D, Tucker T, Opara E, Foster CE, Imagawa D, et al. Strategies to combat hypoxia in encapsulated islet transplantation. Surgery Curr Res. 2016;6(2):259.
Papas KK, et al. Oxygenation strategies for encapsulated islet and beta cell transplants. Adv Drug Deliv Rev. 2019;139:139–56.
Khanna O, et al. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95(2):632–40.
Lembert N, et al. Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplantation. 2005;14(2-3):97–108.
Sigrist S, et al. Induction of angiogenesis in omentum with vascular endothelial growth factor: influence on the viability of encapsulated rat pancreatic islets during transplantation. J Vasc Res. 2003;40(4):359–67.
Trivedi N, et al. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant. 2000;9(1):115–24.
Weaver JD, et al. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials. 2018;172:54–65.
Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci U S A. 2010;107(28):12623–8.
Vaithilingam V, et al. Beneficial effects of desferrioxamine on encapsulated human islets--in vitro and in vivo study. Am J Transplant. 2010;10(9):1961–9.
Opara EC, et al. Design of a bioartificial pancreas(+). J Investig Med. 2010;58(7):831–7.
Smink AM, de Vos P. Therapeutic strategies for modulating the extracellular matrix to improve pancreatic islet function and survival after transplantation. Curr Diab Rep. 2018;18(7):39.
Llacua LA, de Haan BJ, de Vos P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J Tissue Eng Regen Med. 2018;12(2):460–7.
Yan J, Chen F, Amsden BG. Cell sheets prepared via gel-sol transition of calcium RGD-alginate. Acta Biomater. 2016;30:277–84.
Kang SW, et al. The effect of conjugating RGD into 3D alginate hydrogels on adipogenic differentiation of human adipose-derived stromal cells. Macromol Biosci. 2011;11(5):673–9.
Kreeger PK, et al. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–23.
Jo EH, Hwang YH, Lee DY. Encapsulation of pancreatic islet with HMGB1 fragment for attenuating inflammation. Biomater Res. 2015;19:21.
Dang TT, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34(23):5792–801.
Vaithilingam V, et al. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Scientific Reports. 2017;7(1):10059.
Fransson M, et al. Mesenchymal stromal cells support endothelial cell interactions in an intramuscular islet transplantation model. Regen Med Res. 2015;3:1.
Hamilton DC, et al. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. J Tissue Eng Regen Med. 2017;11(3):887–95.
Cantú-Rodríguez OG, et al. Long-term insulin independence in type 1 diabetes mellitus using a simplified autologous stem cell transplant. J Clin Endocrinol Metab. 2016;101(5):2141–8.
Jacobs-Tulleneers-Thevissen D, et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56(7):1605–14.
Basta G, et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes Care. 2011;34(11):2406–9.
Close N, et al. NIH-supported National Islet Transplantation Registry. Cell Biochem Biophys. 2004;40(3 Suppl):9–18.
Acknowledgements
The authors would like to thank all the students and fellows that have worked with Dr. Opara in the microencapsulation technology over the years as their findings have contributed to the knowledge shared in this review article.
Author information
Authors and Affiliations
Contributions
Sittadjody S performed the literature search and generated a draft of the manuscript. Opara EC conceived the idea, generated the outline for the review, and critically edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Artificial Tissues
Rights and permissions
About this article
Cite this article
Sittadjody, S., Opara, E.C. Encapsulation Strategies for Pancreatic Islet Transplantation without Immune Suppression. Curr Stem Cell Rep 7, 49–71 (2021). https://doi.org/10.1007/s40778-021-00190-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00190-w