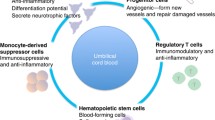
Abstract
Purpose of Review
Paediatric neurological disorders (PNDs) are neurological conditions in infants resulting from injury to the developing nervous system. The neurodevelopmental sequelae in infants with neurological disorders occur from injury to a substantial part of the nervous system. The type of PNDs is classified based on the manifestations, signs and symptoms, and the neurologic core affected by the injury. However, the current prevalence of PNDs underlines the need for optimized and new treatment options.
Recent Findings
PNDs include, but are not limited to, neonatal stroke, cerebral palsy (CP), autism, paediatric status epilepticus (PSE) and spinal cord injury (SCI). Understanding the mechanisms underpinning neuronal necrosis in PNDs has led to the design and trial of potential therapies, including antioxidants, anticonvulsant drugs, mesenchymal stem cells (MSCs), hypothermia and erythropoietin (EPO). With those therapies and other treatment options for PNDs, a significant milestone in the care for children with PNDs has been achieved.
Summary
This review highlights the need for future pre-clinical and clinical studies to investigate a combination therapy of EPO and MSCs for PNDs.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Gonzalez FF, Larpthaveesarp A, McQuillen P, Derugin N, Wendland M, Spadafora R, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44(3):753–8.
Spadafora R, Gonzalez FF, Derugin N, Wendland M, Ferriero D, McQuillen P. Altered fate of subventricular zone progenitor cells and reduced neurogenesis following neonatal stroke. Dev Neurosci. 2010;32(2):101–13.
Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31(5):403–11.
Cameron SH, Alwakeel AJ, Goddard L, Hobbs CE, Gowing EK, Barnett ER, et al. Delayed post-treatment with bone marrow-derived mesenchymal stem cells is neurorestorative of striatal medium-spiny projection neurons and improves motor function after neonatal rat hypoxia-ischemia. Mol Cell Neurosci. 2015;68:56–72.
• Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr Res. 2013;73(1):12–7 This study examines the combination of hypothermia and treatment with erythropoietin in neonatal rat hypoxic-ischaemic. The study found that there was no significant benefit from treatment with either hypothermia or combination therapy.
Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159(5):851–8 e1.
• Rumajogee P, Bregman T, Miller SP, Yager JY, Fehlings MG. Rodent hypoxia-ischemia models for cerebral palsy research: a systematic review. Front Neurol. 2016;7:57 This systematic review compares and discusses the advantages, limitation and the translation value for cerebral palsy research for hypoxia/ischemia models of perinatal brain injury.
Gancia P, Pomero G. Therapeutic hypothermia in the prevention of hypoxic-ischaemic encephalopathy: new categories to be enrolled. Journal of Maternal-Fetal & Neonatal Medicine. 2012;25(sup4):94–6.
Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis Model Mech. 2010;3(11-12):678–88.
Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis. 2016;93:57–63.
• van Velthoven CT, Braccioli L, Willemen HL, Kavelaars A, Heijnen CJ. Therapeutic potential of genetically modified mesenchymal stem cells after neonatal hypoxic-ischemic brain damage. Mol Ther. 2014;22(3):645–54 This study showed that mesenchymal stem cells, modified to secrete specific growth factors is a useful approach for treatment of neonatal HI brain damage. However, necessary care must be taken when selecting the factor to overexpress in the neonatal brain.
Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–24.
Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114.
Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, et al. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61(2):153–8.
Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102(5):1098–106.
Elitt CM, Rosenberg PA. The challenge of understanding cerebral white matter injury in the premature infant. Neuroscience. 2014;276:216–38.
Panigrahy A, Wisnowski JL, Furtado A, Lepore N, Paquette L, Bluml S. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr Radiol. 2012;42(Suppl 1(1)):S33–61.
Kalita J, Kumar V, Misra UK, Bora HK. Memory and learning dysfunction following copper toxicity: biochemical and immunohistochemical basis. Mol Neurobiol. 2018;55(5):3800–11.
Rumbeiha W, Whitley E, Anantharam P, Kim DS, Kanthasamy A. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann N Y Acad Sci. 2016;1378(1):5–16.
Donega V, van Velthoven CT, Nijboer CH, Kavelaars A, Heijnen CJ. The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab. 2013;33(5):625–34.
Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci. 2014;34(1):313–26.
Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, et al. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26(16):4359–69.
Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13(1):127–32.
Nakano N, Nakai Y, Seo TB, Yamada Y, Ohno T, Yamanaka A, et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett. 2010;483(1):57–61.
Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27(4):355–63.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25(7):1342–8.
Petro M, Jaffer H, Yang J, Kabu S, Morris VB, Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. 2016;81:169–80.
• Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291–30 This study study shows that human umbilical cord mesenchymal stem cells can improve cognition of Alzheimer’s disease (AD) mice by decreasing oxidative stress and promoting hippocampal neurogenesis. This comprehensive study also suggest that modulating human umbilical cord mesenchymal stem cells to generate excess neuroprotective factors could provide a viable therapy to treat AD.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24(3):387–93.
Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26(10):1010–21.
Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–52.
Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–89.
Kazanis I. The subependymal zone neurogenic niche: a beating heart in the centre of the brain: how plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed. Brain. 2009;132(11):2909–21.
van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31.
Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–71.
Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23(7):2840–50.
Capilla-Gonzalez V, Lavell E, Quiñones-Hinojosa A, Guerrero-Cazares H. Regulation of subventricular zone-derived cells migration in the adult brain. In: Stem Cell Biology in Neoplasms of the Central Nervous System. Berlin: Springer; 2015. p. 1–21.
Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28(3):545–54.
Otero L, Zurita M, Bonilla C, Rico MA, Aguayo C, Rodriguez A, et al. Endogenous neurogenesis after intracerebral hemorrhage. Histol Histopathol. 2012;27(3):303–15.
Lesny I. History of paediatric neurology: a brief review. J Hist Neurosci. 1995;4(1):25–6.
Chaitanya K, Addanki A, Karambelkar R, Ranjan R. Traumatic brain injury in Indian children. Childs Nerv Syst. 2018;34(6):1119–23.
Seltzer LE, Paciorkowski AR. Genetic disorders associated with postnatal microcephaly: Wiley Online Library; 2014.
Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36(2-3):219–28.
Boulland JL, Lambert FM, Zuchner M, Strom S, Glover JC. A neonatal mouse spinal cord injury model for assessing post-injury adaptive plasticity and human stem cell integration. PLoS One. 2013;8(8):e71701.
Kim JW, Seung H, Kim KC, Gonzales ELT, Oh HA, Yang SM, et al. Agmatine rescues autistic behaviors in the valproic acid-induced animal model of autism. Neuropharmacology. 2017;113(Pt A):71–81.
Perets N, Segal-Gavish H, Gothelf Y, Barzilay R, Barhum Y, Abramov N, et al. Long term beneficial effect of neurotrophic factors-secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav Brain Res. 2017;331:254–60.
Wu H, Wang X, Gao J, Liang S, Hao Y, Sun C, et al. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 2017;173:43–54.
Nitkin CR, Bonfield TL. Concise review: mesenchymal stem cell therapy for pediatric disease: perspectives on success and potential improvements. Stem Cells Transl Med. 2017;6(2):539–65.
Can A. A concise review on the classification and nomenclature of stem cells. Turk J Haematol. 2008;25(2):57–9.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60.
Nakanishi C, Nagaya N, Ohnishi S, Yamahara K, Takabatake S, Konno T, et al. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ J. 2011;75(9):2260–8.
Ha S, Park H, Mahmood U, Ra JC, Suh YH, Chang KA. Human adipose-derived stem cells ameliorate repetitive behavior, social deficit and anxiety in a VPA-induced autism mouse model. Behav Brain Res. 2017;317:479–84.
Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz). 2008;56(1):1–8.
Segers S, Mertes H, de Wert G, Dondorp W, Pennings G. Balancing ethical pros and cons of stem cell derived gametes. Ann Biomed Eng. 2017;45(7):1620–32.
Burns E. More than clinical waste? Placenta rituals among Australian home-birthing women. J Perinat Educ. 2014;23(1):41–9.
Kugler J, Huhse B, Tralau T, Luch A. Embryonic stem cells and the next generation of developmental toxicity testing. Expert Opin Drug Metab Toxicol. 2017;13(8):833–41.
Harris DT. Banking of adipose-and cord tissue-derived stem cells: technical and regulatory issues. In: Biobanking and Cryopreservation of Stem Cells: Springer; 2016. p. 147–54.
Zhang X, Zhang Q, Li W, Nie D, Chen W, Xu C, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res. 2014;92(1):35–45.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst. 2014;30(1):37–46.
Greenfield JP, Ayuso-Sacido A, Schwartz TH, Pannullo S, Souweidane M, Stieg PE, et al. Use of human neural tissue for the generation of progenitors. Neurosurgery. 2008;62(1):21–7.
Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21.
Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr Res. 2005;58(3):600–6.
Sola A, Peng H, Rogido M, Wen TC. Animal models of neonatal stroke and response to erythropoietin and cardiotrophin-1. Int J Dev Neurosci. 2008;26(1):27–35.
Sola A, Rogido M, Lee BH, Genetta T, Wen TC. Erythropoietin after focal cerebral ischemia activates the Janus kinase-signal transducer and activator of transcription signaling pathway and improves brain injury in postnatal day 7 rats. Pediatr Res. 2005;57(4):481–7.
Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10(8):921–39.
Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3(3):a011619.
Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29(4-5):321–30.
Singhi S, Johnston M. Recent advances in perinatal neuroprotection. F1000Res. 2019;8.
Arnaez J, Miranda M, Rinones E, Garcia-Alix A. Whole-body cooling and erythropoietin in neonatal cervical spine injury. Ther Hypothermia Temp Manag. 2019;9(2):159–62.
Lee AYW, Ng SY. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528.
Liu M, Wang AJ, Zhao G, He H, Williams Z, Hu K. Efficacy and safety of erythropoietin for traumatic brain injury: a meta-analysis of randomized controlled trials. medRxiv. 2019:19006601.
Dong H, Li G, Shang C, Yin H, Luo Y, Meng H, et al. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am J Transl Res. 2018;10(3):901–6.
Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, et al. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transplant. 2018;27(2):325–34.
Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31(3):581–91.
Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12(1):1–8.
Liu J, Han D, Wang Z, Xue M, Zhu L, Yan H, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185–91.
Hur JW, Cho T-H, Park D-H, Lee J-B, Park J-Y, Chung Y-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. The Journal of Spinal Cord Medicine. 2016;39(6):655–64.
Jeon SR, Park JH, Lee JH, Kim DY, Kim HS, Sung IY, et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng Regen Med. 2010;7(3):316–22.
Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20(6):806–19.
Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, et al. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013.
Lv Y-T, Zhang Y, Liu M, Ashwood P, Cho SC, Huan Y, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11(1):1–10.
da Silva KOG, da Conceição PS, Portovedo M, Milanski M, Galindo LCM, Guzmán-Quevedo O, et al. Effects of maternal low-protein diet on parameters of locomotor activity in a rat model of cerebral palsy. Int J Dev Neurosci. 2016;52:38–45.
Perlman JM. Intrapartum hypoxic-ischemic cerebral injury and subsequent cerebral palsy: medicolegal issues. Pediatrics. 1997;99(6):851–9.
Fong HD. Trends in cerebral palsy research: Nova Publishers; 2005.
Society. C.P. Cerebral Palsy Society of New Zealand 2020 Available from: https://www.cpsoc.org.nz.
Berry MJ, Jaquiery AL, Oliver MH, Harding JE, Bloomfield FH. Neonatal milk supplementation in lambs has persistent effects on growth and metabolic function that differ by sex and gestational age. Br J Nutr. 2016;116(11):1912–25.
Jia Y, Shi X, Xie Y, Xie X, Wang Y, Li S. Human umbilical cord stem cell conditioned medium versus serum-free culture medium in the treatment of cryopreserved human ovarian tissues in in-vitro culture: a randomized controlled trial. Stem Cell Res Ther. 2017;8(1):1–9.
Wang Z, Luo Y, Chen L, Liang W. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Experimental and therapeutic medicine. 2017;13(6):3613–8.
Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017;18(7):1450.
Lu K, Li H-Y, Yang K, Wu J-L, Cai X-W, Zhou Y, et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):1–11.
Wang X, Cheng H, Hua R, Yang J, Dai G, Zhang Z, et al. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy. 2013;15(12):1549–62.
Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–9.
van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008;59(1):22–33.
Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60(4):386–92.
Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–803.
O’Gorman RL, Bucher HU, Held U, Koller BM, Hüppi PS, Hagmann CF, et al. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138(2):388–97.
Leuchter RH-V, Gui L, Poncet A, Hagmann C, Lodygensky GA, Martin E, et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. Jama. 2014;312(8):817–24.
Rutherford MA, Ramenghi LA, Cowan FM. Neonatal stroke. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F377–84.
Ashwal S, Pearce WJ. Animal models of neonatal stroke. Curr Opin Pediatr. 2001;13(6):506–16.
Harbert MJ, Tam EWY, Glass HC, Bonifacio SL, Haeusslein LA, Barkovich AJ, et al. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol. 2011;26(9):1126–30.
van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012;71(4 Pt 2):474–81.
Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733–43.
Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–7.
Piatt J, Imperato N. Epidemiology of spinal injury in childhood and adolescence in the United States: 1997-2012. J Neurosurg Pediatr. 2018;21(5):441–8.
Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–43.
Basu S. Spinal injuries in children. Front Neurol. 2012;3:96.
Batchelor PE, Skeers P, Antonic A, Wills TE, Howells DW, Macleod MR, et al. Systematic review and meta-analysis of therapeutic hypothermia in animal models of spinal cord injury. PLoS One. 2013;8(8):e71317.
Ruzicka J, Machova-Urdzikova L, Gillick J, Amemori T, Romanyuk N, Karova K, et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant. 2017;26(4):585–603.
Nori S, Ahuja CS, Fehlings MG. Translational advances in the management of acute spinal cord injury: what is new? What is hot? Neurosurgery. 2017;64(CN_suppl_1):119–28.
Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26(5):891–900.
Kemp K, Mallam E, Hares K, Witherick J, Scolding N, Wilkins A. Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts. PLoS One. 2011;6(10):e26098.
da Costa Gonçalves F, Grings M, Nunes NS, Pinto FO, Garcez TNA, Visioli F, et al. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol Lett. 2017;39(4):613–22.
Genc S, Koroglu TF, Genc K. Erythropoietin and the nervous system. Brain Res. 2004;1000(1-2):19–31.
Castillo-Meléndez M, Yan E, Walker DW. Expression of erythropoietin and its receptor in the brain of late-gestation fetal sheep, and responses to asphyxia caused by umbilical cord occlusion. Dev Neurosci. 2005;27(2-4):220–7.
Erbayraktar S, Grasso G, Sfacteria A, Xie Q-W, Coleman T, Kreilgaard M, et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity <em>in vivo</em>. Proceedings of the National Academy of Sciences. 2003;100(11):6741.
Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakç S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci. 2002;99(4):2258–63.
Kumral A, Ozer E, Yilmaz O, Akhisaroglu M, Gokmen N, Duman N, et al. Neuroprotective effect of erythropoietin on hypoxic-ischemic brain injury in neonatal rats. Neonatology. 2003;83(3):224–8.
Campana WM, Misasi R, O’Brien JS. Identification of a neurotrophic sequence in erythropoietin. Int J Mol Med. 1998;1(1):235–76.
Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr. 2014;26(6):668.
Alford EL, Wheless JW, Phelps SJ. Treatment of generalized convulsive status epilepticus in pediatric patients. J Pediatr Pharmacol Ther. 2015;20(4):260–89.
Smith DM, McGinnis EL, Walleigh DJ, Abend NS. Management of status epilepticus in children. J Clin Med. 2016;5(4):47.
Karussis D, Kassis I, Kurkalli BGS, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265(1-2):131–5.
Agadi S, Shetty AK. Concise review: prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells. 2015;33(7):2093–103.
Abdanipour A, Tiraihi T, Mirnajafi-Zadeh J. Improvement of the pilocarpine epilepsy model in rat using bone marrow stromal cell therapy. Neurol Res. 2011;33(6):625–32.
Voulgari-Kokota A, Fairless R, Karamita M, Kyrargyri V, Tseveleki V, Evangelidou M, et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol. 2012;236(1):161–70.
Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84(2-3):238–41.
Shetty AK, Hattiangady B, Shetty G. Intraperitoneal administration of human mesenchymal stem cells restrains status epilepticus induced neurodegeneration and inflammatory reaction in the hippocampus 2014.
Chu K, Jung K-H, Lee S-T, Kim J-H, Kang K-M, Kim H-K, et al. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia. 2008;49(10):1723–32.
Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1996;76(1):105–16.
Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nuñez G, et al. Erythropoietin can induce the expression of bcl-xlthrough stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274(32):22165–9.
Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14(3):494–503.
Rosell DR, Nacher J, Akama KT, McEwen BS. Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122(2):329–48.
Nicolini C, Fahnestock M. The valproic acid-induced rodent model of autism. Exp Neurol. 2018;299(Pt A):217–27.
Giuliani M, Fleury M, Vernochet A, Ketroussi F, Clay D, Azzarone B, et al. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS One. 2011;6(5):e19988.
Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2-3):183–7.
Hell RCR, Costa MMS, Goes AM, Oliveira ALR. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol Dis. 2009;33(2):290–300.
Chang Y-K, Chen M-H, Chiang Y-H, Chen Y-F, Ma W-H, Tseng C-Y, et al. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci. 2011;18(1):1–9.
Siniscalco D, Sapone A, Cirillo A, Giordano C, Maione S, Antonucci N. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012;2012.
Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates: design of a randomized trial. Maternal health, neonatology and perinatology. 2015;1(1):1–9.
Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58(2):297–301.
Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci. 2002;99(14):9450–5.
Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36(8):1672–8.
Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–5.
Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177–87.
Solmaz V, Erdoğan MA, Alnak A, Meral A, Erbaş O. Erythropoietin shows gender dependent positive effects on social deficits, learning/memory impairments, neuronal loss and neuroinflammation in the lipopolysaccharide induced rat model of autism. Neuropeptides. 2020;83:102073.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies and experiments involving human or animal subjects performed by the authors have been previously published and complied with applicable ethical standards as defined in the Helsinki declaration and its amendments, institutional and national research committee standards, and international/national/institutional guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pre-clinical and Clinical Cellular Therapies
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghoghovwia, B.E., Okpe, O., McIntyre, E.A. et al. A Brief Review on Erythropoietin and Mesenchymal Stem Cell Therapies for Paediatric Neurological Disorders. Curr Stem Cell Rep 7, 95–107 (2021). https://doi.org/10.1007/s40778-021-00189-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00189-3