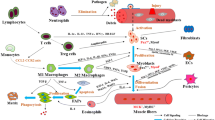
Abstract
Purpose of Review
Skeletal muscle satellite cells (SC) play an important role in the adaptation, repair, and maintenance of muscle. These resident muscle stem cells are regulated by a series of transcription factors known as myogenic regulator factors that orchestrate SC progression from the quiescent state through activation, proliferation, and differentiation, ultimately leading to the contribution of nuclei to muscle fibers. The purpose of this review was to provide an overview of the current state of knowledge on the role of cytokines in regulating muscle SC.
Recent Findings
Upstream effectors of the myogenic regulatory factors include growth factors and cytokines that can influence SC function through autocrine/paracrine signaling delivered via the microvasculature and general circulation, or through cell interactions with other muscle-resident cells and structures.
Summary
Recent evidence describing the relationship between capillaries and muscle SC highlights the importance of this anatomical relationship on SC function in response to physiological stimuli.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170(4):421–35.
Schmalbruch H, Hellhammer U. The number of satellite cells in normal human muscle. Anat Rec. 1976;185(3):279–87.
Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91.
Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 2000;224(2):122–37.
Hawke TJ. Muscle stem cells and exercise training. Exerc Sport Sci Rev. 2005;33(2):63–8.
Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (Bethesda, Md: 1985). 2001;91(2):534–51.
Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development (Cambridge, England). 2011;138(17):3639–46.
Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development (Cambridge, England). 2011;138(17):3625–37.
McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development (Cambridge, England). 2011;138(17):3657–66.
• Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, et al. Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication. FASEB J. 2016;30(6):2266–81. The study demonstrates that the ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs) prompted a greater increase in satellite cell content compared with placebo treatment in the regeneration of skeletal muscle in young healthy men
Beaton LJ, Tarnopolsky MA, Phillips SM. Variability in estimating eccentric contraction-induced muscle damage and inflammation in humans. Can J Appl Physiol = Revue canadienne de physiologie appliquee. 2002;27(5):516–26.
Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242–53.
McKay BR, De Lisio M, Johnston AP, O'Reilly CE, Phillips SM, Tarnopolsky MA, et al. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One. 2009;4(6):e6027.
McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol. 2008;586(Pt 22):5549–60.
Farup J, Rahbek SK, Knudsen IS, de Paoli F, Mackey AL, Vissing K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids. 2014;46(11):2503–16.
Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170–6.
Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, et al. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc. 2013;45(2):230–7.
Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283.
Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One. 2014;9(10):e109739.
Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113(2):99–103.
Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (Bethesda, Md : 1985). 2008;104(6):1736–42.
Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91(8):3024–33.
Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development (Cambridge, England). 2016;143(16):2898–906.
Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22(10):1350–60.
Joanisse S, McKay BR, Nederveen JP, Scribbans TD, Gurd BJ, Gillen JB, et al. Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am J Physiol Regul Integr Comp Physiol. 2015;309(9):R1101–11.
Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2(1):22–31.
Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67.
Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194(1):114–28.
Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol. 2000;278(1):C174–81.
O'Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve. 2008;38(5):1434–42.
Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J Biol Chem. 2000;275(46):35942–52.
Grubb A, Joanisse S, Moore DR, Bellamy LM, Mitchell CJ, Phillips SM, et al. IGF-1 colocalizes with muscle satellite cells following acute exercise in humans. Appl Physiol Nutr Metab = Physiologie appliquee, nutrition et metabolisme. 2014;39(4):514–8.
Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261(11):4828–32.
Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17(4):487–95.
Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522(1–3):156–60.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90.
McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012;26(6):2509–21.
Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, et al. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordrecht, Netherlands). 2014;36(4):9699.
Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. 2014;39:80–6.
Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16(11):1335–47.
Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 2007;35(Pt 5):1295–7.
McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol. 2013;304(8):C717–28.
Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One. 2011;6(3):e17392.
Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–406.
Begue G, Douillard A, Galbes O, Rossano B, Vernus B, Candau R, et al. Early activation of rat skeletal muscle IL-6/STAT1/STAT3 dependent gene expression in resistance exercise linked to hypertrophy. PLoS One. 2013;8(2):e57141.
Donges CE, Duffield R, Smith GC, Short MJ, Edge JA. Cytokine mRNA expression responses to resistance, aerobic, and concurrent exercise in sedentary middle-aged men. Appl Physiol Nutr Metab = Physiologie appliquee, nutrition et metabolisme. 2014;39(2):130–7.
Trenerry MK, Carey KA, Ward AC, Farnfield MM, Cameron-Smith D. Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res. 2008;11(4):717–24.
Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44.
Kurosaka M, Machida S. Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif. 2013;46(4):365–73.
Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31.
Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8(10):e78636.
Orsatti FL, Nahas EA, Orsatti CL, de Oliveira EP, Nahas-Neto J, da Mota GR, et al. Muscle mass gain after resistance training is inversely correlated with trunk adiposity gain in postmenopausal women. J Strength Cond Res. 2012;26(8):2130–9.
Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–48.
Scheele C, Nielsen S, Kelly M, Broholm C, Nielsen AR, Taudorf S, et al. Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6. PLoS One. 2012;7(6):e39657.
Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med. 2005;40(4):432–7.
Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports. 2007;17(5):580–7.
Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (Bethesda, Md : 1985). 2005;98(3):911–7.
Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100(1):93–9.
Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–9.
Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, et al. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003;163(1):203–15.
Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. IL-1beta stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-kappa B. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1249–54.
Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R322–7.
Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136(8):3669–72.
Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280(1):55–63.
Carbo N, Lopez-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, et al. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta. 2001;1526(1):17–24.
Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–33.
Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55(2):241–69.
Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765.
Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol (Baltimore, Md : 1950). 2012;189(7):3669–80.
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7.
Gordon PM, Liu D, Sartor MA, IglayReger HB, Pistilli EE, Gutmann L, et al. Resistance exercise training influences skeletal muscle immune activation: a microarray analysis. J Appl Physiol (Bethesda, Md : 1985). 2012;112(3):443–53.
Prokopchuk O, Liu Y, Wang L, Wirth K, Schmidtbleicher D, Steinacker JM. Skeletal muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 expression and response to strength training. Exerc Immunol Rev. 2007;13:67–75.
Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113(4):483–94.
Lafreniere JF, Mills P, Bouchentouf M, Tremblay JP. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp Cell Res. 2006;312(7):1127–41.
Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–88.
Guerci A, Lahoute C, Hebrard S, Collard L, Graindorge D, Favier M, et al. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2012;15(1):25–37.
Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007 15;120(Pt 4):670–81.
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–42.
Plomgaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev. 2005;11:53–63.
Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163(5):1133–43.
Cantini M, Massimino ML, Bruson A, Catani C, Dalla Libera L, Carraro U. Macrophages regulate proliferation and differentiation of satellite cells. Biochem Biophys Res Commun. 1994;202(3):1688–96.
Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–21.
Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280(6):E1015–21.
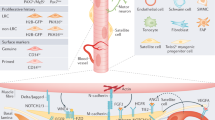
Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18(4):1397–409.
• Nederveen JP, Snijders T, Joanisse S, Wavell CG, Mitchell CJ, Johnston LM, et al. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R85–92. The first study to demonstrate that type II muscle fiber-associated satellite cells are located at a greater distance from its nearest capillary in old compared with young adults.
Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, et al. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle. 2016.
Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270(19):11463–71.
Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, et al. IL-1 stimulates IL-6 production in endothelial cells. J Immunol (Baltimore, Md : 1950). 1989;142(2):549–53.
Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, et al. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42(5):635–42.
Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33.
Margeli A, Skenderi K, Tsironi M, Hantzi E, Matalas AL, Vrettou C, et al. Dramatic elevations of interleukin-6 and acute-phase reactants in athletes participating in the ultradistance foot race spartathlon: severe systemic inflammation and lipid and lipoprotein changes in protracted exercise. J Clin Endocrinol Metab. 2005;90(7):3914–8.
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4.
Eriksson M, Sartono E, Martins CL, Bale C, Garly ML, Whittle H, et al. A comparison of ex vivo cytokine production in venous and capillary blood. Clin Exp Immunol. 2007;150(3):469–76.
Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol. 2003;546(Pt 1):299–305.
Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol (Bethesda, Md : 1985). 2005;98(2):477–81.
Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–8.
Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle fiber composition and capillarization in patients with chronic heart failure: relation to exercise capacity and central hemodynamics. J Card Fail. 1995;1(4):267–72.
Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70.
Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (Bethesda, Md : 1985). 1995;78(6):2033–8.
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–7.
Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordrecht, Netherlands). 2014;36(2):545–7.
Croley AN, Zwetsloot KA, Westerkamp LM, Ryan NA, Pendergast AM, Hickner RC, et al. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol (Bethesda, Md : 1985). 2005;99(5):1872–9.
Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (Bethesda, Md : 1985). 1992;72(5):1780–6.
Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (Bethesda, Md : 1985). 2014;116(8):998–1005.
Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2016 04.
Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27(4):343–56.
Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology. 2017;63(1):91–100.
Acknowledgements
Dr. Gianni Parise was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Grant (1455843), JP Nederveen by a NSERC Canadian Graduate Scholarship (CGS-D). We would like to thank Jessica Blackwood for her assistance in the preparation of the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joshua P. Nederveen, Sophie Joanisse, Tim Snijders, and Gianni Parise declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed by the authors involving human participants were approved by the Hamilton Health Sciences Integrated Research Ethics Board, and conformed to the guidelines outlined in the Declaration of Helsinki. Participants gave their informed written consent prior to inclusion in the study. For animal studies performed by the authors, ethics approval was granted by the McMaster University Animal Research Ethics Board and conformed to the standards established by the Canadian Council on Animal Care.
Additional information
This article is part of the Topical Collection on Cell:Cell Interactions in Stem Cell Maintenance
Rights and permissions
About this article
Cite this article
Nederveen, J.P., Joanisse, S., Snijders, T. et al. The Influence and Delivery of Cytokines and their Mediating Effect on Muscle Satellite Cells. Curr Stem Cell Rep 3, 192–201 (2017). https://doi.org/10.1007/s40778-017-0089-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-017-0089-1