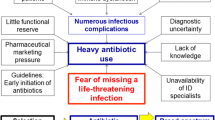
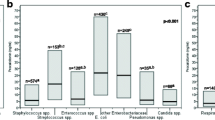
Abstract
Purpose of Review
This review explores the current literature on the diagnosis and management of pediatric culture negative sepsis.
Recent Findings
We explored logistical factors associated with culture negative sepsis, potential diagnostic tools such as biomarkers, and the limited evidence for treatment of culture negative sepsis in children. In the setting of a negative blood culture, but high clinical suspicion for bacterial infections, clinicians look to biomarkers to help guide the use of antibiotics. These biomarkers, however, are not consistently helpful. In general, procalcitonin is superior to CRP for its specificity of a bacterial infection. However, evidence shows this biomarker may better serve to rule out bacterial infection. Other studies suggest procalcitonin may be used as a method to determine the length of antibiotic therapy, but these protocols have not been standardized. Few studies look at appropriate antibiotic duration for culture negative sepsis but suggest it may be adequate to treat for 5–7 days.
Summary
Overall, culture negative sepsis remains difficult to diagnose and has limited guidance for treatment. This is an important area for future research to help limit the misuse of antibiotics in the era of growing antimicrobial resistance.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. (2020) Surviving sepsis campaign international guidelines for management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(1):10–67. https://doi.org/10.1007/s00134-019-05878-6. This article provides expert recommendations for guidelines to in the care of children presenting with sepsis.
Fleiss N, Coggins SA, Lewis AN, Zeigler A, Cooksey KE, Walker LA, et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw Open. 2021;4(2):e2036518. https://doi.org/10.1001/jamanetworkopen.2020.36518.
Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, et al. Sepsis prevalence, outcomes, and therapies (SPROUT) study investigators and pediatric acute lung injury and sepsis investigators (PALISI) network Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–57. https://doi.org/10.1164/rccm.201412-2323OC.
Phua J, Ngerng W, See K, Tay C, Kiong T, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17(5):R202. https://doi.org/10.1186/cc12896.
Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128(5):e1113-1120. https://doi.org/10.1542/peds.2010-2779.
Dietman DE, Fischer GW, Shoenknecht FD. Neonatal Echerichia coli septicemia-bacterial counts in blood. J Pediatr. 1974;85:128–30. https://doi.org/10.1016/s0022-3476(74)80308-2.
Durbin WA, Szymczak EG, Goldmann DA. Quantitative blood cultures in childhood bacteremia. J Pediatr. 1978;92(5):778–80. https://doi.org/10.1016/s0022-3476(78)80151-6.
Kellogg JA, Manzella JP, Bankert DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol. 2000;38(6):2181–5. https://doi.org/10.1128/JCM.38.6.2181-2185.2000.
Isaacman DJ, Karasic RB, Reynolds EA, Kost SI. Effect of number of blood cultures and volume of blood on detection of bacteremia in children. J Pediatr. 1996;128(2):190–5. https://doi.org/10.1016/s0022-3476(96)70388-8.
Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J. 1997;16(4):381–5. https://doi.org/10.1097/00006454-199704000-00009.
Ilstrup DM, Washinton JA. The importance of volume of blood cultured in the detection of bacteremia and fungemia. Diagn Microbiol Infect Dis. 1983;1(2):107–10. https://doi.org/10.1016/0732-8893(83)90039-1.
Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1–94. https://doi.org/10.1093/cid/ciy381.
Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol. 2009;47:3482–5. https://doi.org/10.1128/JCM.02107-08.
Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics. 2007;119:891–6. https://doi.org/10.1542/peds.2006-0440.
Schelonka RL, Chai MK, Yoder BA, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–8. https://doi.org/10.1016/s0022-3476(96)70254-8.
Woodford EC, Dhudasia MB, Puopolo KM, Skerritt LA, Bhavsar M, DeLuca J, Mukhopadhyay S. Neonatal blood culture inoculant volume: feasibility and challenges. Pediatr Res. 2021;90(5):1086–92. https://doi.org/10.1038/s41390-021-01484-9.
Kee PP, Chinnappan M, Nair A, Yeak D, Chen A, Starr M, et al. Diagnostic yield of timing blood culture collection relative to fever. Pediatr Infect Dis J. 2016;35(8):846–50. https://doi.org/10.1097/INF.0000000000001189.
Dien Bard J, McElvania TE. Diagnosis of bloodstream infections in children. J Clin Microbiol. 2016;54(6):1418–24. https://doi.org/10.1128/JCM.02919-15.
Downes KJ, Fitzgerald JC, Weiss SL. Utility of procalcitonin as a biomarker for sepsis in children. J Clin Microbiol. 2020;58(7):e01851-e1919. https://doi.org/10.1128/JCM.01851-19.
Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med. 2008;9:407–13. https://doi.org/10.1097/PCC.0b013e31817285a6.
Lacour AG, Gervaix A, Zamora SA, Vadas L, Lombard PR, Dayer JM, Suter S. Procalcitonin, IL-6, IL-8, IL-1 receptor antagonist and C-reactive protein as identificators of serious bacterial infections in children with fever without localising signs. Eur J Pediatr. 2001;160(2):95–100. https://doi.org/10.1007/s004310000681.
Andreola B, Bressan S, Callegaro S, Liverani A, Plebani M, Da Dalt L. Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J. 2007;26(8):672–7. https://doi.org/10.1097/INF.0b013e31806215e3.
Fernández Lopez A, LuacesCubells C, GarcíaGarcía JJ, FernándezPou J. Spanish Society of Pediatric Emergencies. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J. 2003;22(10):895–903. https://doi.org/10.1097/01.inf.0000091360.11784.21.
Hatherill M, Tibby SM, Sykes K, Turner C, Murdoch IA. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child. 1999;81(5):417–21. https://doi.org/10.1136/adc.81.5.417.
Galetto-Lacour A, Zamora SA, Gervaix A. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referral center. Pediatrics. 2003;112(5):1054–60. https://doi.org/10.1542/peds.112.5.1054.
Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics. 2008;122(4):701–10. https://doi.org/10.1542/peds.2007-3503.
Gomez B, Bressan S, Mintegi S, Da Dalt L, Blazquez D, Olaciregui I, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815–22. https://doi.org/10.1542/peds.2011-3575.
Milcent K, Faesch S, Gras-Le Guen C, Dubos F, Poulalhon C, Badier I, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62–9. https://doi.org/10.1001/jamapediatrics.2015.3210.
Kuppermann N, Dayan PS, Levine DA, Vitale M, Tzimenatos L, Tunik MG, et al. Febrile infant working group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342–51. https://doi.org/10.1001/jamapediatrics.2018.5501.
Chaurasia S, Anand P, Sharma A, Nangia S, Sivam A, Jain K, et al. Procalcitonin for detecting culture-positive sepsis in neonates: a prospective, multicenter study. Neonatology. 2023;120(5):642–51. https://doi.org/10.1159/000529640.
Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J. 1999;18(10):875–81. https://doi.org/10.1097/00006454-199910000-00008.
Lautz AJ, Dziorny AC, Denson AR, O’Connor KA, Chilutti MR, Ross RK, et al. Value of procalcitonin measurement for early evidence of severe bacterial infections in the pediatric intensive care unit. J Pediatr. 2006;179:74-81.e2. https://doi.org/10.1016/j.jpeds.2016.07.045.
Han YY, Doughty LA, Kofos D, Sasser H, Carcillo JA. Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. Pediatr Crit Care Med. 2003;4:21–5. https://doi.org/10.1097/00130478-200301000-00004.
Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin And Survival Study (PASS) Group. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–58. https://doi.org/10.1097/CCM.0b013e31821e8791.
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77. https://doi.org/10.1086/510393.
Campion M, Scully G. Antibiotic use in the intensive care unit: optimization and de-escalation. J Intensive Care Med. 2018;33(12):647–55. https://doi.org/10.1177/0885066618762747.
Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children’s hospital. Pediatrics. 2011;128(6):1062–70. https://doi.org/10.1542/peds.2010-3589.
Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237–45. https://doi.org/10.1093/cid/cit498.
Gao Y, Liu M, Yang K, Zhao Y, Tian J, Pernica JM, Guyatt G. Shorter versus longer-term antibiotic treatments for community- acquired pneumonia in children: a meta-analysis. Pediatrics. 2023;151(6):e2022060097. https://doi.org/10.1542/peds.2022-060097.
Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196–203. https://doi.org/10.1542/peds.2009-2948.
Tamma PD, Turnbull AE, Milstone AM, Lehmann CU, Sydnor ER, Cosgrove SE. Ventilator-associated tracheitis in children: does antibiotic duration matter? Clinical Infectious Disease. 2011;52(11):1324–2133. https://doi.org/10.1093/cid/cir203.
• Singh N, Gray JE. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J Perinatol. 2021;41(10):2488–94. https://doi.org/10.1038/s41372-021-01110-w. This is a retrospective before-after cohort study demonstrating a reduction in antibiotics in the NICU with the removal of routine CRP collection. With this reduction in antibiotics used for early onset sepsis, they did not find an increase in partially treated cases. Patients with high suspicion of sepsis were still treated, but there was a reduction in antibiotics used days 3-6 when suspicion was low.
Astorga MC, Piscitello KJ, Menda N, Ebert AM, Ebert SC, Porte MA, Kling PJ. Antibiotic stewardship in the neonatal intensive care unit: effects of an automatic 48-hour antibiotic stop order on antibiotic use. J Pediatric Infect Dis Soc. 2019;8(4):310–6. https://doi.org/10.1093/jpids/piy043.
Downes KJ, Weiss SL, Gerber JS, Klieger SB, Fitzgerald JC, Balamuth F, et al. A pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in Sepsis (OASIS) Study. J Pediatr Infect Dis Soc. 2017;6:134–41. https://doi.org/10.1093/jpids/piw023.
•• Morowitz MJ, Katheria AC, Polin RA, Pace E, Huang DT, Chang CH, Yabes JG. The NICU Antibiotics and Outcomes (NANO) trial: a randomized multicenter clinical trial assessing empiric antibiotics and clinical outcomes in newborn preterm infants. Trials. 2022;23(1):428. https://doi.org/10.1186/s13063-022-06352-3. This is a multicenter, randomized control trial assessing early antibiotic exposure side effects/outcomes in extremely low birth weight infants. The goal is to determine if the current practice of early antibiotics outweighs the risks of antibiotic exposure.
Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5. https://doi.org/10.1016/j.jpeds.2011.05.033.
Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–7. https://doi.org/10.1016/j.jpeds.2011.02.035.
•• Downes KJ, Fitzgerald JC, Schriver E, Boge CLK, Russo ME, Weiss SL, et al. Implementation of a pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in Sepsis (OASIS) II Study. J Pediatr Infect Dis Soc. 2020;9:36–43. https://doi.org/10.1093/jpids/piy113. This study suggests that the use of biomarkers, procalcitonin and CRP may be supportive when low in decisions to stop antibiotics in PICU patients with SIRS but no source of infection. However, the authors biomarker-based algorithm did not decrease overall antibiotic prescribing in patients with SIRS.
Stocker M, van Herk W, el Helou S, Dutta S, Fontana MS, Schuerman FABA, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns.). Lancet. 2017;390:871–81. https://doi.org/10.1016/S0140-6736(17)31444-7.
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–74. https://doi.org/10.1016/S0140-6736(09)61879-1.
Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505. https://doi.org/10.1164/rccm.200708-1238OC.
Cantey JB, Wozniak PS, Pruszynski JE, Sanchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(10):1178–84. https://doi.org/10.1016/S1473-3099(16)30205-5.
Wehrenberg K, Mitchell M, Zembles T, Yan K, Zhang L, Thompson N. Antibiotic treatment duration for culture-negative sepsis in the pediatric intensive care unit. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e249. https://doi.org/10.1017/ash.2023.502.
Author information
Authors and Affiliations
Contributions
K.W. wrote the main manuscript text. M.M. and N.T. revised the work critically. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wehrenberg, K., Mitchell, M. & Thompson, N. The Diagnostic and Therapeutic Challenges of Culture Negative Sepsis. Curr Treat Options Peds (2024). https://doi.org/10.1007/s40746-024-00293-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40746-024-00293-6